Review
HJOG 2022, 21 (1), 1-14 | doi: 10.33574/hjog.0401
Konstantina Papadatou, Paraskevas Perros, Nikolaos Thomakos, Dimitrios Haidopoulos, Alexandros Rodolakis, Vasilios Pergialiotis
First department of obstetrics and gynecology, Section of gynecologic oncology, Alexandra Hospital, National and Kapodistrian University of Athens, Greece
Correspondence: Konstantina Papadatou, Lour 2-4 str, Athens, Greece, P.C. 11528, email: konstantina.papadatou@gmail.com
Abstract
Cervical cancer is the fourth most common gynecologic malignancy worldwide with an estimated incidence of approximately 570000 cases worldwide in 2018. Despite the advances that were made in the primary and secondary prevention strategies the last decades, its actual prevalence seems to reach a negative plateau which is attributed to the limited acceptance of vaccination as well as the resources that are available in several low income countries. In the present article we review novel biomarkers that seem to interfere in the pathophysiology of the disease and can, thus, be used as tools for the early identification of patients at risk as well as biomarkers of disease response to the various treatment strategies.
Introduction
Globally, cervical cancer is considered to be one of the most common gynecological malignant tumors, highly occurred especially in developing countries. Its incidence ranks the second one among female tumor causes and it is also estimated to consist the cause of approximately 527,600 new cases and 265,700 deaths annually1.
According to strong epidemiological evidence, a necessary, but not sufficient, condition for cervical cancer to be present is the persistent infection with carcinogenic human papillomavirus (HPV) genotypes2. It is of vital importance to note that the majority of HPV genotypes are not highly carcinogenic. In fact, there are currently 226 HPV genotypes classified according to their carcinogenicity, as denoted by the International Agency for Research on Cancer (IARC-WHO), with a subset of these being identified as high risk3.
The main histological subtypes of cervical cancer are the squamous cell carcinoma and the adenocarcinoma, with the first representing the 80% of all cervical cancer cases4. The unfavorable prognosis of cervical cancer patients is contributed to the tendency of tumor cells to infiltrate surrounding tissues and spread, resulting in both lymphatic and distant metastasis5.
Therefore, it is necessary to study proteins that are differently expressed in cervical cancer cells and investigate the key regulatory factors of this expression. Indeed, a large number of such proteins is available nowadays. Among them, the serum squamous cell carcinoma antigen (SCC Ag), the carcinoembryonic antigen (CEA) and the oncoproteins E6 and E7 are of particular importance. Τhese biomarkers seem very promising. However, apparently not in every single case their application is sufficient in clinical practice. Moreover, the development of novel biomarkers may provide not only opportunities to distinguish high risk patients, but also enable the development of new therapeutic targets and strategies, improving the survival rate of the patients.
Tumor biomarkers
Oncology biomarkers are defined as easily accessible and measurable biologic substances for screening and monitoring of occult tumors.
SCC Ag
Serum squamous cell carcinoma antigen (SCC Ag) belongs to the family of serine and cysteine protease inhibitors and represents a subfraction of tumor – associated antigens related to squamous cell carcinoma6. It is normally present in cervix epithelium with an increased expression in dysplastic lesions and cervical squamous cell carcinoma. Kato and Torigoe, concretely, demonstrated SCC Ag to have positive rates in squamous cell carcinoma in 2,44% of carcinoma in situ cases, in 22,2% in FIGO stage I cases, in 56,7% in stage II cases, in 76,4% in stage III cases and, lastly, also in 76,4% in stage IV cases7. Thus, nowadays SCC Ag consists a widely used biomarker for cervical cancer. Actually, pretreatment elevated serum SCC levels have found to have an independent prognostic value, being associated with advanced stage of disease, extensive tumor, regional nodal involvement as well as lymphovascular and deep stromal infiltration8. It must be noted that a serum level of ≥ 8,6mg/ml seem to be able to predict lymph node metastasis with a positive predictive value of 100%9.
Moreover, SCC Ag seems to have an important role in predicting the response to treatment in cervical cancer patients. More specifically, either the elevated SCC Ag value itself or the rate of its reduction during concurrent chemoradiotherapy (CCRT) may predict tumor response6.
Last but not least, the levels of SCC Ag may contribute in monitoring cervical cancer patients and detecting recurrent or progressive disease in squamous cell cervical cancers10. Oh et al. demonstrated the rate of 2 ng/ml to be the optimal cutoff value of SCC Ag for detecting tumor relapse11. The presence, also, of a plateau in SCC Ag levels or even its increasing rates after or during primary treatment suggest tumor persistence or progressive disease. According to Kawagushi et al.12, the optimal cutoff point for the posttreament SCC Ag level one month after the completion of CCRT is 1,15 ng/ml, with values ≥ 1,15ng/ml concluding in a 3-year overall survival rate (OS) of 36,6% and a 3-year progression-free survival (PFS) rate of 19,5% in cervical cancer patients.
CYFRA 21-1
Serum CYFRA 21-1 consists a fragment of cytokeratin 19 and is an established tumor marker for a variety of malignant tumors, and for cervical cancer as well. It tends to be positive more frequently in squamous cell carcinoma in comparison to the histological subtype of adenocarcinoma13.
The existence of positive CYFRA 21-1 rates seem to correlate with the appearance of the disease, and the increase of its levels with the progression of the clinical stage of cervical cancer14. CYFRA 21-1 also tends to decline during irradiation treatment, with negative values in disease-free patients after the therapy. Therefore, CYFRA 21-1 positivity at the end of the radiation therapy is considered to indicate reliably remaining distant metastasis.
Lastly, there are many reports that have demonstrated the mainly supporting role of CYFRA 21-1 to SCC Ag. Not only patients with cervical cancer negative for SCC Ag are found to be positive for CYFRA 21-1, but also the coordinated monitoring of the two markers could contribute to the quality of the follow-up of the patients with the early detection of possible recurrences.
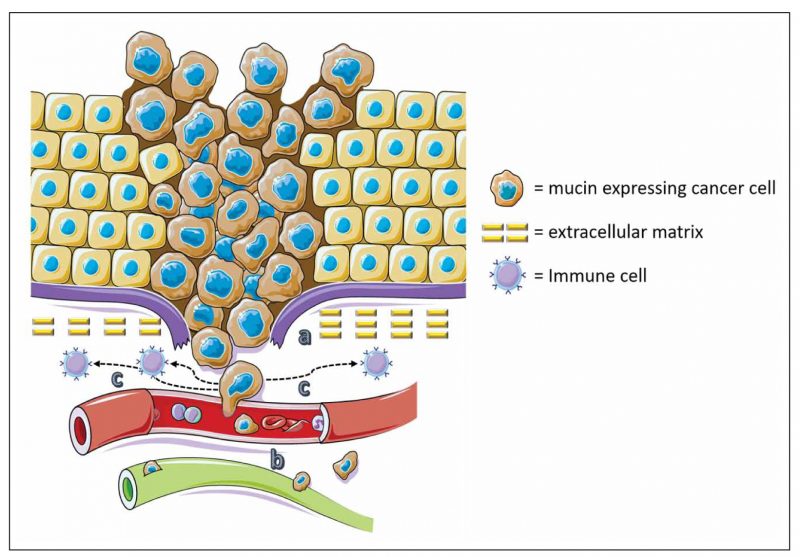
Figure 1. Tumours use mucins for invasion, metastasis and protection. a) Tumour cells use the anti-adhesive effect of mucins to detach from the tumour mass and surrounding stroma and invade. b) Tumours cells use the adhesive effect of mucins to attach to endothelia and invade blood vessels. c) Tumour cells also use mucins to escape immune surveillance acting as barriers against T-cells.
CEA
Carcinoembryonic antigen (CEA) is a glycoprotein with a molecular weight of approximately 200.000 Daltons isolated first by Gold and Freeman in 1965. It is found in normal fetal gastrointestinal tissue and normally, it is present at very low concentrations in adult plasma, but its values can be increased in the presence of many tumors.
Studies have focused on CEA for over 40 years. Lo Gerfo et al. (1971) were the first to report elevated serum CEA in patients with gynecological malignancies. Disaia et al. (1977)15 studied a group of patients with invasive squamous cell carcinoma of the cervix and found that there was a progressive increase in the percentage of patients with positive CEA values correlating with advancing stage of the disease from 26% in stage I to 88% in stage II. Moreover, it is shown that serum CEA has the potential of prognosticating cervical cancer patients, being a reliable marker for the presence of lymph node metastasis. More specifically, CEA values are found to be elevated in 73,3 % of the patients with pelvic lymph involvement, as well as in 100% of them with paraaortic lymph nodal involvement15.
Thee significant patterns of alteration in serum CEA levels are descripted16. Firstly, the pattern of free disease, in which there is a disappearance of CEA in serum after treatment17, consisting a favorable prognostic indicator in cervical cancer. Actually, plasma concentrations decrease to normal levels within 4-8 weeks following treatment. Secondly, in residual cancer cases, CEA is persistently detected in serum, possibly in high levels. Finally, in 85% of the recurrent cases positive CEA values are shown15. Clinical recurrence can be detected during follow up of patients when CEA reappears in the serum, possibly up to 6 months before clinical detection.
MUC 16
As aforementioned, tumor markers have a vital role in diagnosing, prognosticating and monitoring patients with cervical cancer. Mucine 16 (MUC16), previously known as cancer antigen 125 (CA-125) consists a mucinous transmembrane glycoprotein with an average molecular weight between 3-5 million Da18, encoded by the MUC16 gene. It is actually an antigenic determinant recognized by a monoclonal antibody (OC125) which was raised using an ovarian cancer cell line as an immunogen19.
CA – 125
CA-125 is an extensively investigated biomarker for monitoring epithelial ovarian cancer, as well as other pathological and normal tissues of Müllerian origin. Its application in cervical cancer consists in the prediction of prognosis, in particular in cervical adenocarcinoma20, as elevated levels are detectable in 20-75% of these patients. In contrast, only 13% to 21% of women with squamous cell carcinoma of the cervix are found to present an augmentation in CA-125 rate17, making SCC to be up to date the tumor marker of choice in squamous tumors.
Moreover, the serum levels of CA-125 are used to predict preoperatively the presence of lymph node metastases, also mainly in cervical adenocarcinoma20. In one study which included 50 patients with metastatic cervical cancer17, it has been reported that CEA consists a better biomarker for diagnosing recurrences, as 40 patients presented an augmentation of CEA serum levels in contrast to CA-125 levels, which were elevated in only 15 out of 50 cases. However, despite a specificity of 90%, CEA sensitivity in cervical cancer as a single marker was not found to exceed 15%. It was shown that sensitivity in indicating cancer virulence and possible relapse was improved while using a combination of CA-125 and CEA biomarkers.
PCNA, Ki – 67
The rate of cell proliferation in a tumor is generally considered to be of prognostic importance. The two proliferation antigens which have been most widely studied are proliferating cell nuclear antigen (PCNA), which is expressed during the G1 and early S phases of the proliferative cell cycle, and Ki- 67, which is expressed during the G2 and mitotic phases of the cycle21.
Proliferating cell nuclear antigen (PCNA) constitute a 29 kDa protein known to be activated by the E7 oncoprotein of high-risk human papillomavirus (HPV). Despite the large number of studies analyzing PCNA expression in cervical intraepithelial neoplasia (CIN) and cervical cancer lesions, the data still remain controversial. Actually, PCNA expression seems to increase in parallel with the grade of CIN, with a major up-regulation upon progression to CIN322. Intense PCNA expression has been also found in cervical carcinoma patients, with a number of studies to demonstrate a possible association of its rates with disease recurrence and lymph node metastases23,24. However, another study with series of 150 patients failed to prove the existence of prognostic value of PCNA levels in cervical cancer22.
As a gene capable of promoting cell proliferation, abnormal expression of Ki-67 protein usually indicates also abnormal cell proliferation. One study that included paraffin specimens from 64 patients with atypical Thinprep Cytology Test (TCT) screening followed by colposcopic biopsy in Shanghai, China, noted Ki-67 expression to be detectable in LSIL, HSIL, and in squamous cervical cancer (SCC) cases but not in normal cervical tissues25. The rate of this expression was significantly positively correlated with the degree of cervical lesions. Hanprasertpong et al26 found an elevated Ki-67 expression in 81.3% of SCC cases, while the latter was reported to consist an independent prognostic factor for 5-year recurrence-free survival in multivariate analysis. Finally, it should be underlined that Ki-67 and p16 tend to be combined in order to identify patients with high risk of SCC, as Ki-67 alone is shown to be also expressed in non-cancerous proliferative cervical tissues in a rate of 18,18%, which in fact has a diagnostic significance leading possibly to a misdiagnosis25.
Oncoproteins
Oncoproteins E6, E7
Oncoproteins E6 and E7, known also as “HPV oncoplayers”, are the major driving force for cervical carcinogenesis. They are responsible from the initiation point of tumor development including the maintenance of continuous proliferative signaling, the escape of tumor suppressors, and activation of telomerase, to the induction of angiogenesis and invasion to metastatic stages. In other words, E6 and E7 contribute in the establishment and successful progression of cervical cancer.
More concretely, E6 binds to p53, via the ubiquitin ligase E6-AP (E6-associated protein). This results in p53 degradation, preventing cells from p53-mediated apoptosis. Oncoprotein E6, also, activates the telomere lengthening enzyme telomerase and binds with PDZ proteins, resulting in their inactivation.
Moreover, E7 binds to pRb and its related pocket proteins p107 and p130 (negative cell cycle regulators), resulting in pRb inactivation. Then, pRb inactivation results in activation of E2F transcription factors, cell cycle progression/cell proliferation and, finally, suspension of apoptosis.
As aforementioned, since these oncogenes are the major carcinogenic factors, E6 and E7 targeting could help targeting specifically cervical cancer cells. Consequently, they turn out to consist the most effective drug candidates for HPV-infected cancers, including cervical cancer. It should be noted that a variety of treatments have been applied using E6 and E7 targeting, all of which have their own pros and cons. Nowadays, combinatorial approach consists the form of clinical practice. This could result in better ways to combat the high-mortality index of cervical cancer worldwide27.
Last but not least, it has been demonstrated that high expression of HPV E6/E7 mRNA could be a good candidate as a diagnostic biomarker to triage ASCUS superseding HPV DNA. Also, p16/Ki-67 immunocytochemistry is suggested to be a good tool to triage ASCUS, but it reduces the sensitivity of diagnosis when improves the diagnostic specificity28.
Oncogenes
c – MYC
The cellular oncogene c-MYC is frequently found amplified and overexpressed in cervical cancer. Several studies have shown MYC activation at premalignant stages, indicating that MYC detection might be used to assess dysplastic lesions29. Golijow et al. have performed PCR based detection of MYC amplification on histological and cytological specimens. It was demonstrated that MYC levels increase with lesion grade at premalignant stages. In a consecutive study, Abba et al. showed a tight correlation between MYC expression and HPV16 infection at pre-invasive stages, indicating different oncogenic properties of different HR-HPV types.
Angiogenic agents
Αs known, human solid tumors contain hypoxic regions with a considerably lower oxygen tension than healthy tissues. Lack of oxygen (hypoxia) constitutes an indicator of solid tumor formation, as well as an independent prognostic factor in a variety of malignant tumors. Cancer cells adapt to hypoxia by stabilising HIF-α isoforms, which increase the transcription of several genes. Among the genes regulated by HIF are enzymes that have a role in proliferation, invasion, metastasis and metabolism of tumour cells. Numerous cancer studies have, lately, investigated HIF-1α as an important cancer drug target. In the case of cervical cancer, HIF-1α is able to affect proliferation, apoptosis, cell cycle and invasion30.
HIF – 1a
It is noted that increased HIF-1a expression in advanced uterine cervical carcinoma induces expression of VHL and is not associated with epigenetic regulation.
RQ-PCR and Western blot analysis were used to compare HIF-1a and VHL transcript and HIF-1a protein levels in normal and cancerous tissues. According to the results, significantly higher levels of HIF-1a and VHL transcript (p<0.0001 & p=0.0042, respectively) and HIF-1a protein (p=0.0037) levels were found in cancerous tissue compared to normal tissue samples. There were no significant differences between transcript and protein levels in patient groups with different tumor stage and histological grade. We also did not observe DNA methylation in the HIF-1a and VHL promoter region in either control or cancerous tissues samples (results not shown). A statistically significant association was found between HIF-1A and VHL expression (Spearma correlation. coefficient=0.515, p=0.003)
However, less is known about HIF-2α, another HIF-α isoform. One previous study demonstrated that the HIF isoforms (HIF-1α and HIF-2α) have divergent effects on invasion, metastasis, metabolism and formation of lipid droplets in human breast cancer cells.Therefore, the present study comprehensively investigated the function of HIF-2α in cervical cancer cell line CaSki and compared the function of HIF-1α and HIF-2α on proliferation, cell cycle, apoptosis, cell invasion and cell autophagy31.
VEGF, PD – ECGF
Other promising targets which play critical roles in tumor growth and angiogenesis are the vascular endothelial growth factor (VEGF) and the platelet-derived endothelial cell growth factor (PD-ECGF). They are highly associated with the tumor microvessel count32. VEGF is upregulated by E6, independent from p53, and its overexpression is considerded to be an early marker of CIN and correlated linearly with lesion grade.
The levels of VEGF-A in patients with bulky tumor, pelvic lymph-node involvement (PLNI), and parametrial infiltration (PI) are found to be significantly higher than those in patients without these factors (P = 0.022, P = 0.020, and P = 0.0013, respectively). Moreover, the overall survival (OS) of patients with high VEGF-A and VEGFR-2, defined by median levels, is considered lower than the OS of patients with low levels of VEGF-A and VEGFR-2 (P = 0.014, P = 0.012, respectively)33. Multivariate analysis revealed that PLNI, serum VEGF-A levels, and serum VEGFR-2 levels were independent prognostic factors for OS (hazard ratio for VEGF-A 3.42, 95% CI 1.07-13.2; hazard ratio for VEGFR-2 6.37, 95% CI 1.59-43.5)34.
The expression level of another angiogenic factor, the platelet-derived endothelial cell growth factor (PD-ECGF) has also been associated with the tumor microvessel count in cases of cervical and ovarian cancer, as well as uterine carcinoma34. Although, its expression in metastatic lymph node lesions is extremely poor.
Replication/ complex proteins
MCM
The Minichromosome Maintenance Complex (MCM) constitutes a replication complex. The expression of the genes 2, 4, 5 and 7 is found to be augmented in cervical dysplasia. Concretely, its expression increases during the progression of the disease from LSIL to HSIL, with the highest expression to be in cases of SCC35. As far as MCM2 gene is concerned, it seems to have the highest fold change in SCC compared to normal cervix. Immunohistochemically, MCM2 protein is localised in the nuclei of basal cells of normal cervical epithelium and dysplastic-neoplastic cells of CIN and SCC.
TYMS
Thymidylate Synthetase (TYMS) encodes thymidine synthase (TS), an important enzyme that catalyzes the synthesis of pyrimidines, is important for DNA synthesis and repair, and also a target of fluorouracil medicines. Multiple studies have shown that the expression level of TYMS may be related to the sensitivity of radiotherapy and chemotherapy for cervical cancer36. Suzuki, Tsukagoshi, Saga, Ohwada, and Sato (1999) compared the immunohistochemical level of TS and the survival of 66 patients with stage IIIb cervical cancer, showing that the 5-year survival rate with high and low level of TS was 36.8% and 87.2%, respectively. Therefore, high TS may be associated with poor prognosis of cervical cancer.
WRN
The Werner gene (WRN) codes for a DNA helicase that contributes to genomic stability and has been identified as the gene responsible for progeria. Recent studies37 have shown reduced WRN expression, and even suppression, due to aberrant DNA hypermethylation, in cancer cells. Several studies have, also, shown that WRN inactivation increases the anticancer effect of CPT-11, a chemotherapy drug. CPT-11 acts on the covalent complex of topoisomerase I (Top-I) and DNA, inhibiting DNA replication and causing strand breaks.
Anti-inflammatory agents
APOC1
Apolipoprotein C1 (APOC1) is a member of the apolipoprotein family that normally transports lipids, stabilizes the lipoprotein structure and regulates various pathological processes. Recently, apolipoproteins are found to be overexpressed in several neoplasms. APOC1, more accurately, is noted to act as an oncogene in cervical cancer. A late study5 demonstrated an up-regulated expression of APOC1 in cases of cervical carcinomas (p < 0.05) as well as a restrained tumorigenicity of cancer cells after the knockdown of this factor. Furthermore, it is suggested that APOC1 affects the capacity of cancer to metastasize, and maybe plays a key role in the progression mechanism of the disease, thus providing a direction for clinical treatment. Consequently, APOC1 could serve as an important biomarker and therapeutic target for cervical cancer.
COX – 2
Cyclooxygenase-2 (COX-2) is the inducible isoform of cyclooxygenase and catalyzes the conversion of arachidonic acid to prostaglandins. COX-2 and the prostaglandin cascade play a major role in carcinogenesis, including the appearance of cervical carcinoma. More specifically, this isoform, when overexpressed in cervical cancer patients, it is suspected to promote angiogenesis and tissue invasion of the tumor, as well as resistance to apoptosis38. Moreover, COX-2 dependent prostaglandin release can suppress antigen presentation and immune activation in cancer. Associations between the intensity of immunostaining and reduced survival rates have been noted, with overall 5-year survival rates to be 57% for COX-2 positive patients and 83% for COX-2 negative patients, regardless of the histological subtype7. Last but not least, the inhibition of COX-2 tends to increase the radiosensitivity of cervical cancer, making this factor a candidate biomarker for the prediction of the response of malignancy in the subsequent therapy.
IGF
In addition, the insulin-like growth factor (IGF) is organized in a complex network which consists, among others, of three ligants, IGF-I, IGF-II and Insulin. IGF system is found to have a significant role in the development and progression of cervical cancer. According to Harris et al.39, the IGF axis seems to have an influence on the natural history of oncogenic HPV and the progression of cervical malignancy. A case-control study, also, conducted on women with SIL and cervical cancer40, concluded that elevated levels of IGF-I and IGF-II were linked with tumor progress and a higher possibility of recurrence, making these factors promising targets for cervical cancer invasion and metastasis control.
CD34
As fas as CD34 antigen is concerned, it is a transmembrane glycoprotein and consists a highly sensitive biomarker for endothelial cell differentiation, extensively studied in tumor angiogenesis41. In cervical carcinoma cases, it has been demonstrated that anti-CD34 antibody is associated not only with pathoanatomical features indicative of a poorer prognosis, such as undifferentiated tumor, lymphatic vessel invasion and lymph node involvement, but also with a greater risk of recurrence. In a study, more specifically, that included 62 patients diagnosed with invasive cervical carcinoma42, CD34 was noted to determine a higher microvessel density and to correlate with an also higher angiogenic activity.
CYCLINS
Cyclin represents a family of regulatory proteins that controls the progression of a cell through the cell cycle, whereas there are several different cyclins active in different parts of the latter. The expression of cyclins has been analyzed in cervical malignancy and precancer situations43. Therefore, cyclin D1 expression is found to be increased in low-grade lesions, while cyclins A, B and E are found to be overexpressed in premalignant cervical lesions. Finally, cyclin E is strongly associated with HPV induced cellular abnormalities.
CD 109
Finally, Cluster of Differentiation 109 (CD109) consists a glycoprotein which, lately, emerges as a potential biomarker and a therapeutic target for squamous cell carcinomas, including cervical ones. In the study go Zhang et al. a significantly higher CD109 expression has been reported in these cases compared with endometrial adenocarcinomas, normal cervix and endometrium7,44.
Other
CD44v6, XRCC1
CD44v6 consists an isoform of CD44 family adhesion molecules. Although its functions remain unclear, it plays an important role in the growth and metastasis development in malignant tumors of different origins, such as cervical cancer. In cervical carcinoma, strong correlations between CD44v6 expression and lymphovascular space invasion (LVSI) and regional lymph node metastases have been reported45. Poor prognosis in patients with CD44v6 tumor positivity (both early and late cervical cancer) is noted, and it is also described as an independent prognostic factor in early stage disease. Despite the fact that CD44v6 is generally considered to be associated with a shortened overall survival (OS), Saegusa et al46. had found it to correlate with the histological type of tumor, especially with SCC, but no with clinico-pathological factors or OS45. X-ray repair cross-complementing protein 1 (XRCC1) is a major gene involved in the efficient repair of DNA single-strand breaks formed by exposure to ionizing radiation and alkylating agents. Polymorphisms in DNA repair genes associated with repair efficiency against DNA damage may predispose in cancer susceptibility47.
mTOR
The mechanistic target of rapamycin (mTOR) is a kinase that serves as a core component of two protein complexes, mTOR complex 1 and mTOR complex 2, which regulate distinct cellular processes. Among its functions, mTOR regulates cell growth and proliferation, as well as cell motility, autophagy and survival. Activation of mTOR signaling pathway contributes to the development of variant tumors, inclusive of cervical carcinoma. Consecutively, inhibition of mTOR could represent a potential therapeutic strategy for them, with the mTOR cascade to be a promising target fro intervention48.
In closing, an elevated expression of CD44v6, XRCC1 gene polymorphism and a high level of phosphorylated mTOR, all three are associated with a poor response of cervical cancer to chemotherapy in patients treated with cisplatin-based neoadjuvant chemotherapy. Thus, they may have a significant role as markers to predict the efficacy of the treatment before its administration and the survival of the cervical cancer patients7.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2018 Nov;68(6):394-424.
2. Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011 Sep;6(9): 10.2217/fmb.11.87.
3. Chrysostomou AC, Kostrikis LG. Methodologies of Primary HPV Testing Currently Applied for Cervical Cancer Screening. Published: 19 November 2020.
4. Wang D , Li Q, Li Y, Wang H. The role of MCM5 expression in cervical cancer: Correlation with progression and prognosis. Biomed Pharmacother. 2018 Feb;98:165-172.
5. Shi X, Wang J, Dai S, Qin L, Chen JZY. Apolipoprotein C1 (APOC1): A Novel Diagnostic and Prognostic Biomarker for Cervical Cancer. Onco Targets Ther. 2020 Dec 15;13:12881-12891.
6. Fu J, Wang W, Wang Y, Liu C, Wang P. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol. 2019; 14: 146.
7. Iida M, Banno K, Yanokura M, Nakamura K, Adachi M, Nogami Y, Umene K, Masuda K, Kisu I, Iwata T, Tanaka K, Aoki D. Candidate biomarkers for cervical cancer treatment: Potential for clinical practice. Mol Clin Oncol. 2014 Sep;2(5):647-655.
8. Yim EK, Park JS. Biomarkers in Cervical Cancer. Biomarker Insights 2006:1 215–225.
9. Bolger BS, Dabbas M, Lopes A, Monaghan JM. Prognostic value of preoperative squamous cell carcinoma antigen level in patients surgically treated for cervical carcinoma. Gynecol Oncol. 1997 May;65(2):309-13.
10. Chao X, Fan J, Song X, You Y, Wu H, Wu M, Li L. Diagnostic Strategies for Recurrent Cervical Cancer: A Cohort Study. Front Oncol. 2020; 10: 591253.
11. Οh J, Bae JY. Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance. Obstet Gynecol Sci. 2018 May; 61(3): 337–343.
12.Kawaguchi R, Furukawa N, Kobayashi H, Asakawa I. Posttreatment cut-off levels of squamous cell carcinoma antigen as a prognostic factor in patients with locally advanced cervical cancer treated with radiotherapy. J Gynecol Oncol. 2013 Oct; 24(4): 313–320.
13. Hamzaoui A, Thomas P, Castelnau O, Roux N, Roux F, Kleisbauer JP. Usefulness of longitudinal evaluation of Cyfra 21-1 variations in advanced lung cancer monitoring. Lung Cancer. 1997 Mar;16(2-3):191-202.
14. Suzuki Y, Nakano T, Atsuko Abe T.O, Morita S, Tsujii H. Serum CYFRA 21-1 in cervical cancer patients treated with radiation therapy. J Cancer Res Clin Oncol (2000) 126:332±336.
15. Chandana G, Vishwanathan B, Malik MA, Reddy SK, Raghavendra DS. Role of Serum CEA as Tumor Marker for Predicting Presence of Pelvic and Paraaortic Lymph Node Metastasis in SCC of Uterine Cervix. J Cancer Prev Curr Res. 2017 Aug; 8(2): 00274.
16. Carcinoembryonic Antigen (CEA) In Cervical Neoplasia (Review) june, 2011. Contemporary OB/GYN.
17. Kaiser J, Javeed A, Hajira F, Suryanarayana G.R. Significance of CA125 and CEA as biomarkers in assessing progress of treatment for cervical cancer. IJPSR. 2021 June; 12(6).
18. Felder M, Kapur A, Gonzalez-Bodquet J, Horibata S,Heinz J, Albrecht R, Fass L, Kaur J,Hu K, Shojaei H, Whelan RJ, Patankar MS. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014; 13: 129.
19. Nolen BM, Lokshin AE. Ovarian Carcinoma Cell Line- an overview. Biomarkers in Toxicology, 2014.
20. Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015 May 20;445:7-11.
21. Kim TH, Han JH, Shin E, Noh JH, Kim HS, Song YS. Clinical Implication of p16, Ki-67, and Proliferating Cell Nuclear Antigen Expression in Cervical Neoplasia: Improvement of Diagnostic Accuracy for High-grade Squamous Intraepithelial Lesion and Prediction of Resection Margin Involvement on Conization Specimen. J Cancer Prev. 2015 Mar; 20(1): 70–77.
22. Branca M, Ciotti M, Giorgi C, Santini D, Bonito L, Costa S, Benedetto A, Bonifacio D, Bonito P, Paba P, Accardi L, Syrjaven S, Favalli C, Syrjanen K. Up-regulation of proliferating cell nuclear antigen (PCNA) is closely associated with high-risk human papillomavirus (HPV) and progression of cervical intraepithelial neoplasia (CIN), but does not predict disease outcome in cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2007 Feb;130(2):223-31.
23. Tjalma W, Weyler J, Pollefliet C. The evaluation of proliferative activity in CIN III and micro invasive cervical cancer and its role in recurrence. Eur J Obstet Gynecol Reprod Biol 2001;94:270-5.
24. Maeda MY, Simoes M, Wakamatsu A. Relevance of the rates of PCNA, Ki-67 and p53 expression according to the epithelial compartment in cervical lesions. Pathologica 2001; 93:189-95.
25. Qin S, XU L, Yang R, Meng Y, Qiu L. Ki-67 and P16 proteins in cervical cancer and precancerous lesions of young women and the diagnostic value for cervical cancer and precancerous lesions. Oncol Lett. 2019 Aug; 18(2): 1351–1355.
26. Hanprasertpong J, Tungsinmunkong K, Chichareon S. Correlation of p53 and Ki-67 (MIB-1) expressions with clinicopathological features and prognosis of early stage cervical squamous cell carcinomas. J Obstet Gynaecol Res 36: 572-580, 2010.
27. Pal A, Kundu R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front Microbiol. 2019; 10: 3116.
28. Ran C, Zhu Y, Yang L, Zhang X, Liu L, Wang Z, Jiang D. Prognostic and diagnostic validity of p16/Ki-67, HPV E6/E7 mRNA, and HPV DNA in women with ASCUS: a follow-up study. Virol J. 2019; 16: 143.
29. Wentzensen N, Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007;23(4): 315-30.
30. Łuczak MW, Roszak A, Pawlik P, Kedzia H, Lianeri M, Jagodzinski PP. Increased expression of HIF-1A and its implication in the hypoxia pathway in primary advanced uterine cervical carcinoma. Oncol Rep. 2011 Nov;26(5):1259-64.
31. Jiang L, Shi S, Shi Q, Zhang H, Hu R, Wang M. Similarity in the functions of HIF1α and HIF2α proteins in cervical cancer cells. Oncol Lett. 2017 Nov; 14(5): 5643–5651.
32. Sawada M, Oishi T, Komatsu H, Sato S, Chikumi J, Nonaka M, Kudoh A, Osaku D, Harada T. Serum vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 as prognostic biomarkers for uterine cervical cancer. Int J Clin Oncol. 2019 Dec;24(12):1612-1619.
33. Campo JM, Prat A, Gil-Moreno A, Perez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008 Sep;110(3 Suppl 2):S72-6.
34. Iida M, Banno KJ, Yanokura M, Nakamura K, Adachi M, Nogami Y, Umene K, Masuda K, Kisu I, Iwata T, Tanaka K, Aoki D. Candidate biomarkers for cervical cancer treatment: Potential for clinical practice. Mol Clin Oncol. 2014 Sep;2(5):647-655.
35. Kaur G, Balasubramaniam SD, Lee YJ, Balakrishnan V, Son CE. Minichromosome Maintenance Complex (MCM) Genes Profiling and MCM2 Protein Expression in Cervical Cancer Development. Asian Pac J Cancer Prev. 2019 Oct 1;20(10):3043-3049.
36. Yang HJ, Xue J, Li J, Wan L, Zhu Y. Identification of key genes and pathways of diagnosis and prognosis in cervical cancer by bioinformatics analysis. Mol Genet Genomic Med. 2020 Jun;8(6):e1200.
37. Masuda K, Banno K, Yanokura M, Tsuji K, Kobayashi Y, Kisu I, Ueki A, Yamagami W, Nomura H, Tominaga E, Susumu N, Aoki D. Association of epigenetic inactivation of the WRN gene with anticancer drug sensitivity in cervical cancer cells. Oncol Rep. 2012 Oct;28(4):1146-52.
38. Liu B, Qu L, Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015 Nov 5;15:106.
39. Harris TG, Burk RD, Yu H, Minkoff H, Massad LS, Watts DH, Zhong Y, Gange S, Kaplan RC, Anastos K, Levine AM, Moxley M, Xue X, Fazzari M, Palefsky JM, Strickler HD. Insulin-like growth factor axis and oncogenic human papillomavirus natural history. Cancer Epidemiol Biomarkers Prev. 2008 Jan;17(1):245-8.. 2008 Jan;17(1):245-8.
40. Serrano ML, Romero A, Cendales R, Sanchez-Gomez M, Bravo MM. Serum levels of insulin-like growth factor-I and -II and insulin-like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancer. Biomedica. 2006 Jun;26(2):258-68.
41. Acute C, Acute E, Zugun-Eloae FI, Carasevici E. Neoangiogenesis in cervical cancer: focus on CD34 assessment. Rom J Morphol Embryol. 2010;51(2):289-94.
42. Vieira SC, Silva BB, Pinto GA, Vassallo J, Moraes NG, Santana JOI, Santos LG, Carvasan GAF, Zeferino LC. CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol Res Pract. 2005;201(4):313-8.
43. Wentzensen N, Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007; 3(4): 315-30.
44. Qi R, Dong F, Liu Q, Murakumo Y, Liu J. CD109 and squamous cell carcinoma. J Transl Med. 2018 Apr 6;16(1):88.
45. Bouda J, Boudova L, Hes O, Havir M, Tempfer C, Kohlberger P, Svoboda T, Rokyta Z, Speiser P. CD44v6 as a prognostic factor in cervical carcinoma FIGO stage IB. Anticancer Res. Jan-Feb 2005;25(1B):617-22.
46. Saegusa M, Hashimura M, Machida D, Okayasu I. Down- regulation of CD44 standard and variant isoforms during the development and progression of uterine cervical tumours. J Pathol. 1999 Jan;187(2):173-83.
47. Zeng X., Zhang Y, Yue T, Zhang T, Wang J, Xue Y, Ruifang A. Association between XRCC1 polymorphisms and the risk of cervical cancer: a meta-analysis based on 4895 subjects. Oncotarget. 2017 Jan 10; 8(2): 2249–2260.
48. Faried LS, Faried A, Kanuma T, Sano T, Nakazato T, Tamura T, Kuwano H, Minegishi T. Predictive and prognostic role of activated mammalian target of rapamycin in cervical cancer treated with cisplatin-based neoadjuvant chemotherapy. Oncol Rep. 2006 Jul;16(1):57-63.