Review
HJOG 2023, 22 (2), 57-62 | doi: 10.33574/hjog.0525
Nikolaos Machairiotis1, Vasilios Pergialiotis2, Konstantinos Vlassis3, Theodoros Troupis3, Nikolaos Vrachnis1
13rd Department of Obstetrics and Gynecology, Attikon Hospital, National and Kapodistrian University of Athens, Greece
21st Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, “Alexandra” General Hospital, Athens, Greece
3Department of Anatomy, School of Medicine, faculty of health sciences, national and Kapodistrian University of Athens, Greece
Correspondence: Nikolaos Machairiotis, 3rd Department of Obstetrics and Gynecology, Attikon Hospital, National and Kapodistrian
University of Athens, Greece, e-mail: nikolaosmachairiotis@gmail.com
Abstract
Neurotrophins have been previously mentioned as potential factors that may alter human growth and result in fetal growth restriction (FGR). As neural growth factors, neurotrophins promote brain development and plasticity and have a significant effect on the synaptic transmission, although the actual mechanisms of their action have not been completely investigated. Several articles have been published in this field denoting the importance of neurotrophins as biomarkers of detection of fetuses with fetal growth restriction. Their findings are contradictory and the purpose of the present systematic review is to summarize existing evidence and provide recommendations for future research.
Keywords: Neyrotrophin, growth restriction
Introduction
Fetal growth restriction (FGR) is a relatively common antenatal pathology that is encountered in approximately 3-7% of pregnancies1 and refers to the inability of the fetus to retain its genetically attributed growth potential. Several factors contribute to the occurrence of FGR including congenital infections, malnutrition, anemia and chronic diabetes mellitus.2 Two distinct subgroups of FGR are identified, namely the asymmetrical FGR which refers to the majority (70-80%) of cases and the symmetrical group which is encountered in 20-30% of pregnancies complicated by the condition.3 The asymmetrical form of FGR is identified during late pregnancy and is mainly the result of disproportionate growth of the fetus which preserves the central fetal blood flow, therefore, enabling brain growth, together reducing the blood flow to the liver and reducing the abdominal circumference. Most cases that are complicated by asymmetrical FGR have already developed preeclampsia and the reduced blood flow that is observed in the context of altered vasculogenesis may significantly impair placental oxygen uptake.4 The symmetrical form, although less prevalent, is significantly more complex as it refers to conditions that affect pregnancy during the first trimester and results in complex antenatal and postnatal pathology that is the result of the condition that affected the intrauterine growth.
Several mechanisms have been proposed as potential pathophysiological triggering factors of FGR, however, still several gaps exist that require further investigation. Among those, neurotrophins have been previously proposed as potential factors that may alter human growth and result in FGR.5 These proteins are well known growth factors that are primarily expressed in the brain, but can be identified in peripheral tissues as well.6,7 As neural growth factors, neurotrophins promote brain development and plasticity and have a significant effect on the synaptic transmission, although the actual mechanisms of their action have not been completely investigated.8 Four proteins comprise the neurotrophin family, namely the brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), NT-3, and NT-4. It is believed that they modulate several functions as their action relies in two distinct receptors; the Trk and the p75 receptor, an effect that is believed to allow an increased degree of freedom in terms of neural modulation. Specifically, the activation of the Trk pathway seems to promote cell survival, whereas activation of the p75 receptor induces apoptosis, as this receptor is a member of the tumor necrosis factor (TNF) receptor family (Figure 1).9
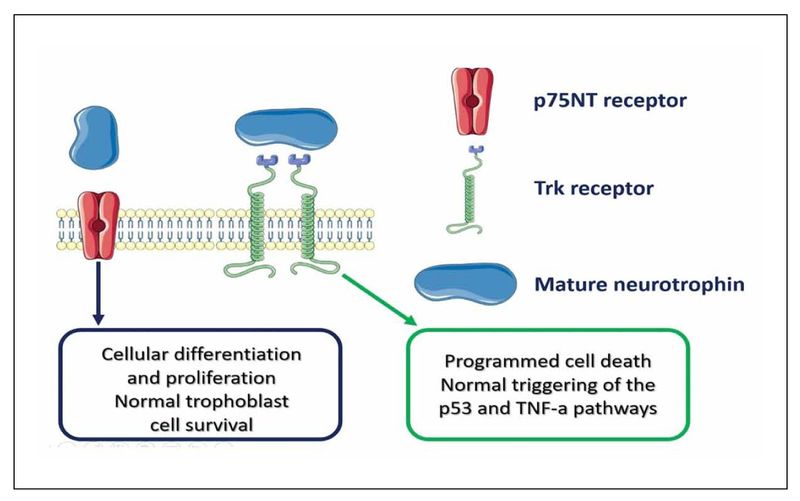
Figure 1.
Research in the field of antenatal pathology remains to date relatively rare, although there are scarce reports that suggest an association with growth restriction. Of note, previous researchers indicated that NGF is important during the early stages of placental development as it is expressed by cytotrophoblasts, syncytiotrophoblasts, chorionic mesodermal cells and decidual cells.10 In the present communication letter we summarize the available evidence that correlates neurotrophins with fetal growth restriction and provide directions for future research.
Methods
Design and eligibility criteria
To detect new studies published after the publication of the previous systematic review11 we systematically searched the literature considering current recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 Eligibility criteria for the inclusion of studies were predetermined. We chose to include observational studies and randomized trials that compared differences in the expression of neurotrophins as well as potential gene polymorphisms that may affect their functionality among cases with FGR and healthy control pregnancies. The review was designed to include all studies that were published in the Medline (1966–2023), Scopus (2004–2023), Clinicaltrials.gov (2008–2023), EMBASE (1980-2023), Cochrane Central Register of Controlled Trials CENTRAL (1999-2023) databases. The date of last search was set at February, 15th 2023.
Results
Evidence related to the significant contribution of human placental neurotrophic factors on fetal development has been derived since 2013 by Dhobale et al who described that preterm fetuses have significantly lower mRNA expression of Brain-derived neurotrophic factor (BDNF) in placental tissue as well as lower level of nerve growth factor (NGF).13 The authors also observed that despite the lack of a correlation of those proteins with preterm delivery, a positive association between mRNA levels and protein expression was evident, therefore, leading to the assumption of a potential correlation that could not be detected due to the small sample size that was used. They also attributed their findings to potential epigenetic differences compared to control placentas that were retrieved from pregnancies delivered at term and underlined the potential implications of altered fetal programming.
It should be noted that the expression of neurotrophins in the placental varies significantly among its different regions, as, according to Sahay et al their levels are significantly higher in the central fetal region compared to the central maternal and peripheral fetal/maternal region.14 Taking this information into consideration it becomes easily understandable that maternal levels of those cytokines does not necessarily reflect the fetal levels, and more importantly gaps in the available research may be attributed to the lack of standardized methodology in the uptake of those proteins.
Noting this, it becomes easily understandable that a direct extrapolation of these findings to fetal growth restriction should not be regarded as prudent, evidence from Malamitsi-Puchner et al suggests that the levels of NGF in term fetal growth restricted fetuses are significantly lower compared to term fetuses of average birthweight for their gestational age (AGA).15 Of note, in this study the levels of neurotrophins were measured in neonates and their mothers at day 1 and 4 and no correlation with growth restriction was observed in BDNF, NT-3 and NT-4 among FGR and AGA cases.
Amniotic fluid levels reflect better the actual intravascular fetal levels of cytokines. In this context Antonakopoulos et al observed the association between BDNF levels in amniotic fluid during the second trimester of pregnancy and fetal development and observed significant differences among severely growth restricted fetuses (<3 percentile) compared to AGA fetuses.16 Even the evaluation of amniotic fluid levels of neurotrophins is not, however, entirely free of bias as Flock et al reported that high maternal BMI and low maternal blood BDNF may differentiate the limit of detection of amniotic fluid BDNF throughout gestation.17
Possibly the best way to observe potential differences among growth restricted and AGA fetuses is to review cord blood levels of neurotrophins. To date, this has been investigated only in term pregnancies by Briana et al in pregnancies complicated by gestational diabetes mellitus with the authors mentioning the absence of a specific correlation with fetal growth percentiles.18
It remains relatively, unknown how these differences in neurotrophin levels among normal and growth restricted infants may hinder human development and specifically neurodevelopment as current evidence remains extremely scarce. Richter et al observed altered methylations in the neurodevelopmental DNA among fetal growth restricted fetuses that were subject to brain-sparing.19 Specifically, the authors reported significant hypermethylation at a binding site for cyclic adenosine monophophate response element binding protein (CREB) of BDNF promoter exon 4 and hypomethylation at an HRE located within the neurotrophic tyrosine kinase, receptor, type 2 (NTRK2) promoter.
This information seems to be very important as it may guide future research by helping to establish therapeutic models in growth restricted fetuses. Experimental studies suggest that supplementation with taurine, a naturally occurring sulfur-containing amino acid that enhances neural growth, increased the ratio neurons to glial cells and prevented gliosis in the differentiation of neural stem cells.20,21 The whole process was mediated by the protein kinase A-cyclic adenosine monophosphate (cAMP) and the response element protein-brain derived neurotrophic factor (PKA-CREB-BDNF) signaling pathway.
Conclusion
Neurotrophins seem to play an important role in embryo development, an effect that is equally distributed during three trimesters of pregnancy. To date, evidence correlating these proteins with fetal growth restriction remains scarce and extremely heterogeneous; hence, it remains unknown if they may serve as predictive factors during early pregnancy, or even if they may become targets of future therapeutic strategies, as indicated by experimental animal studies. Future research must focus in specific populations, including those with gestational diabetes mellitus and preeclampsia, as their expression seems to significantly differentiate compared to healthy pregnancies. Moreover, investigation of the actual site to be targeted from future researchers is necessary as current knowledge indicates differences in expression even among samples from different placental locations.
Disclosure
The authors report no conflict of interest
Funding
None to disclose for all authors.
References
- Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 2009;6 Suppl 3(332-6
- Maulik D. Fetal growth restriction: the etiology. Clin Obstet Gynecol 2006;49(2):228-35, doi:10.1097/00003081-200606000-00006
- Shrivastava D, Master A. Fetal Growth Restriction. J Obstet Gynaecol India 2020;70(2):103-110, doi:10.1007/s13224-019-01278-4
- Maulik D. Fetal growth compromise: definitions, standards, and classification. Clin Obstet Gynecol 2006;49(2):214-8, doi: 10.1097/00003081-200606000-00004
- Sahay A, Kale A, Joshi S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020;83(102075, doi:10.1016/j.npep.2020.102075
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24(677-736, doi:10.1146/annurev.neuro.24.1.677
- Rush RA, Zhou X-F. Neurotrophin Immunohistochemistry in Peripheral Tissues. In: Neurotrophin Protocols. (Rush RA. ed.) Humana Press: Totowa, NJ; 2001; pp. 21-29.
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nature Reviews Neuroscience 2003;4(4):299-309, doi:10.1038/nrn1078
- Pathare-Ingawale P, Chavan-Gautam P. The balance between cell survival and death in the placenta: Do neurotrophins have a role? Systems Biology in Reproductive Medicine 2022;68(1):3-12, doi:10.1080/19396368.2021.1980132
- Toti P, Ciarmela P, Florio P, et al. Human placenta and fetal membranes express nerve growth factor mRNA and protein. Journal of Endocrinological Investigation 2006;29(4):337-341, doi:10.1007/BF03344105
- Santoro A, Travaglino A, Inzani F, et al. Prognostic Value of Chemotherapy Response Score (CRS) Assessed on the Adnexa in Ovarian High-Grade Serous Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics 2022;12(3):633
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology 2009;62(10):e1-34, doi:10.1016/j.jclinepi.2009.06.006
- Dhobale MV, Pisal HR, Mehendale SS, et al. Differential expression of human placental neurotrophic factors in preterm and term deliveries. International Journal of Developmental Neuroscience 2013;31(8):719-723, doi:https://doi.org/10.1016/j.ijdevneu.2013.09.006
- Sahay AS, Sundrani DP, Wagh GN, et al. Neurotrophin levels in different regions of the placenta and their association with birth outcome and blood pressure. Placenta 2015;36(8):938-943, doi:https://doi.org/10.1016/j.placenta. 2015.06.006
- Malamitsi-Puchner A, Nikolaou KE, Economou E, et al. Intrauterine growth restriction and circulating neurotrophin levels at term. Early Hum Dev 2007;83(7):465-9, doi:10.1016/j.earlhumdev.2006.09.001
- Antonakopoulos N, Iliodromiti Z, Mastorakos G, et al. Association between Brain-Derived Neurotrophic Factor (BDNF) Levels in 2(nd) Trimester Amniotic Fluid and Fetal Development. Mediators Inflamm 2018;2018(8476217, doi:10.1155/2018/8476217
- Flöck A, Odainic A, Dolscheid-Pommerich R, et al. High maternal BMI and low maternal blood BDNF may determine the limit of detection of amniotic fluid BDNF throughout gestation: Analysis of mother-fetus trios and literature review. PLoS One 2022;17(3):e0265186, doi:10.1371/journal.pone.0265186
- Briana DD, Papastavrou M, Boutsikou M, et al. Differential expression of cord blood neurotrophins in gestational diabetes: the impact of fetal growth abnormalities. J Matern Fetal Neonatal Med 2018;31(3):278-283, doi:10.1080/14767058.2017.1281907
- Richter AE, Bekkering-Bauer I, Verkaik-Schakel RN, et al. Altered neurodevelopmental DNA methylation status after fetal growth restriction with brain-sparing. J Dev Orig Health Dis 2022;13(3):378-389, doi:10.1017/s2040174421000374
- Fang Q, Liu J, Chen L, et al. Taurine improves the differentiation of neural stem cells in fetal rats with intrauterine growth restriction via activation of the PKA-CREB-BDNF signaling pathway. Metab Brain Dis 2021;36(5):969-981, doi:10.1007/s11011-021-00672-0
- Liu J, Liu Y, Wang XF, et al. Antenatal taurine supplementation improves cerebral neurogenesis in fetal rats with intrauterine growth restriction through the PKA-CREB signal pathway. Nutr Neurosci 2013;16(6):282-7, doi:10.1179/1476830513y.0000000057