Research
HJOG 2024, 23 (1), 69-77| doi: 10.33574/hjog.0551
Konstantinos Ravanos1, Kyriaki Mitta2, Ioannis Tsakiridis2, Eleni-Markella Chalkia-Prapa2, Yannis Prapas1, Nikolaos Prapas1, Apostolos Athanasiadis2, Themistoklis Dagklis2
1IAKENTRO fertility Center, Thessaloniki, Greece
2Third Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Greece
Correspondence: Tsakiridis Ioannis, 49 Konstantinoupoleos str., 54642, Thessaloniki, Greece, Tel: +30 2313312120, Fax: +30 2310 992950, e-mail: igtsakir@auth.gr
Abstract
Introduction: Temporal global trends of sperm quality remain a matter of debate. It is widely believed that exposure to endocrine disruptors during fetal life can damage testicular function, cause testicular cancer and impair reproduction.
Materials and methods: This was a retrospective, cohort study using data from a single IVF center located in Thessaloniki, Greece, during the period 2004-2013; including male partners of infertile Greek women, undergoing oocyte donation treatment for the first time. Local polynomial smoothing was used to assess visually possible trends in the data. One-way ANOVA was used for the comparison of means of variables of interest between years. The Pearson Chi-squared and the Chi-squared test for trend were employed to test for the independence and trend respectively of the occurrence of concentrations <20 million/ml and the year of study.
Results: A total of 1113 participants were analyzed. The mean sperm concentration increased significantly between 2004 and 2012 (39.5million/ml versus 59.1million/ml; p<0.001), but without forming a visible upward trend; the mean total motility remained statistically unchanged (56% versus 49.8%; p=0.239), as well as the percentage of sperm concentration of less than 20million/mL (23.5% versus 24.4%; p=0.249), whereas the mean of rapid progressive motile sperm decreased significantly (41.1% versus 30%; p<0.001), forming a visible downward trend.
Conclusions: Sperm motility and annual percentage of oligospermic males presenting with a sperm concentration of less than 20 million/mL showed an absence of significant change and temporal trend. Rapid progressive motile sperm deteriorated significantly, revealing a visible time-related downward trend.
Keywords: semen quality, sperm concentration, sperm decline, infertility
Introduction
An ongoing, still unresolved scientific debate regarding temporal trends of semen quality was triggered in the 1990s1. A meta-analysis that evaluated 61 published papers regarding semen quality parameters of 14947 samples concluded that during a period of 50 years, between 1938 and 1991, global sperm counts of non-infertile men declined by 50%, that is 1% per year2.
The impact of the Carlsen study was massive, as for the first time it was evident that a continuous deterioration of semen quality could potentially jeopardize human fertility2; at the same time, significant criticism was raised regarding the high heterogeneity of the participants3, the lack of adjustment for confounding factors that are known to cause great variations in semen parameters4,5, the variability of the laboratory techniques employed for semen analysis6, as well as the improper statistical tools employed to interpret the data7.
Regarding temporal trends of semen quality parameters in the Greek population there is only one retrospective study published in 1996, reporting a statistically significant decrease in the sperm count of male partners of couples investigated for sub-fertility in the greater area of Athens, over a period of 17 years8. To our knowledge, this early publication was followed by a paucity of relevant data and until now, no other attempt or publication has updated data with respect to possible trends in semen quality among the Greek population.
This study aimed to investigate whether semen parameters among male partners of infertile Greek couples, undergoing oocyte donation treatment in a single fertility center for the first time, exhibit significant changes over a period of 10 years.
Materials and Methods
This retrospective study was conducted in a single private fertility center (IAKENTRO) in Thessaloniki, Greece; over a period of ten years (January 2004–December 2013). The present study was approved by the ethical committee of the Institutional Board regarding the protection of personal data analyzed (approval decision No 5/2014 dated 3/2/2014).
The study population were male partners of totally infertile Greeks, who were seeking oocyte donation treatment for the first time and for whom information about age and fresh semen quality parameters was available. Couples were excluded if the male partner was diagnosed with obstructive azoospermia following surgical sperm retrieval, if he was treated in the past either for cryptorchidism or for genital cancer, if the semen provided was not freshly ejaculated by masturbation and if they have been using procreative drugs and steroids of any kind in the past, as these factors have been reported to impair semen quality. For each individual, a preliminary spermogram and a semen culture were always available prior to IVF treatment, following the fertility investigation of the couple the preceding year.
All semen samples included in the study were obtained by masturbation and ejaculation into a wide-mouth sterile plastic cup, in a private room attached to the semen laboratory facilities, following strictly 72 hours of sexual abstinence. Each patient included had a negative semen culture and was treated for prophylaxis with 500mg/day of azithromycin during the abstinence period. Each patient was provided with one semen sample on the day of the donor’s oocyte retrieval, which was examined by the same certified biologist within one (1) hour after ejaculation employing standard laboratory procedures in compliance with the 4th edition of the World Health Organization (WHO) laboratory manual9. At least thirty (30) minutes were provided for sample liquefaction on a heated stage at 37oC. Determination of sperm concentration and motility status was performed with the Makler counting chamber which was prewarmed at 37oC under a light microscope[10].
The primary outcome of the study was sperm concentration (millions per milliliter). Secondary outcomes of the study were total sperm motility (WHO a+b+c, %), sperm rapid progressive motility (WHO a, %), and the annual count of patients presenting with a sperm concentration of less than 20 million/ml which defines oligospermia according to WHO published guidelines9.
Normality for continuous variables was assessed using the Kolmogorov-Smirnov test. Data are described as means ± standard deviations for continuous variables and as frequencies followed by proportions for categorical variables. Local polynomial smoothing was used in order to assess visually possible trends in the data. Results were compared with the horizontal line that was defined by the mean value of the studied variable. One-way ANOVA with Tukey-Kramer post-hoc tests was used for the comparison of means of variables of interest between years. Data for variables measured on a continuous scale were depicted using box plots and scatter plots. The Pearson Chi-squared test and the Chi-squared test for trend were employed in order to test for the independence and for trend respectively of the occurrence of concentrations <20 million/ml and the year of study. Results are followed by bar charts and mosaic plots. P-values less than 0.05 were considered statistically significant. The IBM SPSS 21.0 (IBM Corp., Armonk, NY) and JMP 11.0 (SAS Inst., Cary, NC) statistical packs were used for data analysis.
Results
Overall, 1113 male partners of infertile couples seeking oocyte donation treatment for the first time, fulfilled the inclusion criteria and entered the study group analysis with a mean (±SD) age of 43.8 (±6.1) years. During the study period, the annual size of samples varied between 45 and 183 (mean: 111) couples. Semen parameters and analysis are shown in Table 1.
The mean sperm concentration during the study period was 46.8 (SD: 34.9) million/mL, with the highest value found in 2012 (59.1 million/mL; SD: 33.6 million/mL) and the lowest value found in 2004 (39.5 million/mL; SD: 27.2 million/mL). Overall, a statistically significant increase was observed in sperm concentration between 2004 and 2012 (39.5 million/ml versus 59.1million/ml; p<0.001) within the decade of the study (Figure 1). Local polynomial smoothing was used in order to assess visually possible trends in the data which were depicted with scatter plots. Results were compared with the mean of the variable, revealing no temporal trends of sperm concentration within the decade of the study. Annual variations of the sperm concentration within the years of the study were obvious but these differences failed to provide a clear visible upward trend, even though a progressive increase was evident but with intervals and no consistency (Figure 2). On the other hand, some annual variations of the sperm concentrations were significantly different when compared with others. In fact, lower values of sperm concentration were encountered in the first years of the study (2004; 2005; 2006) and significantly higher values were encountered in the next years (2008; 2009; 2012). No significant difference was observed between the rest of the years (2007; 2011; 2013) (Figure 1).
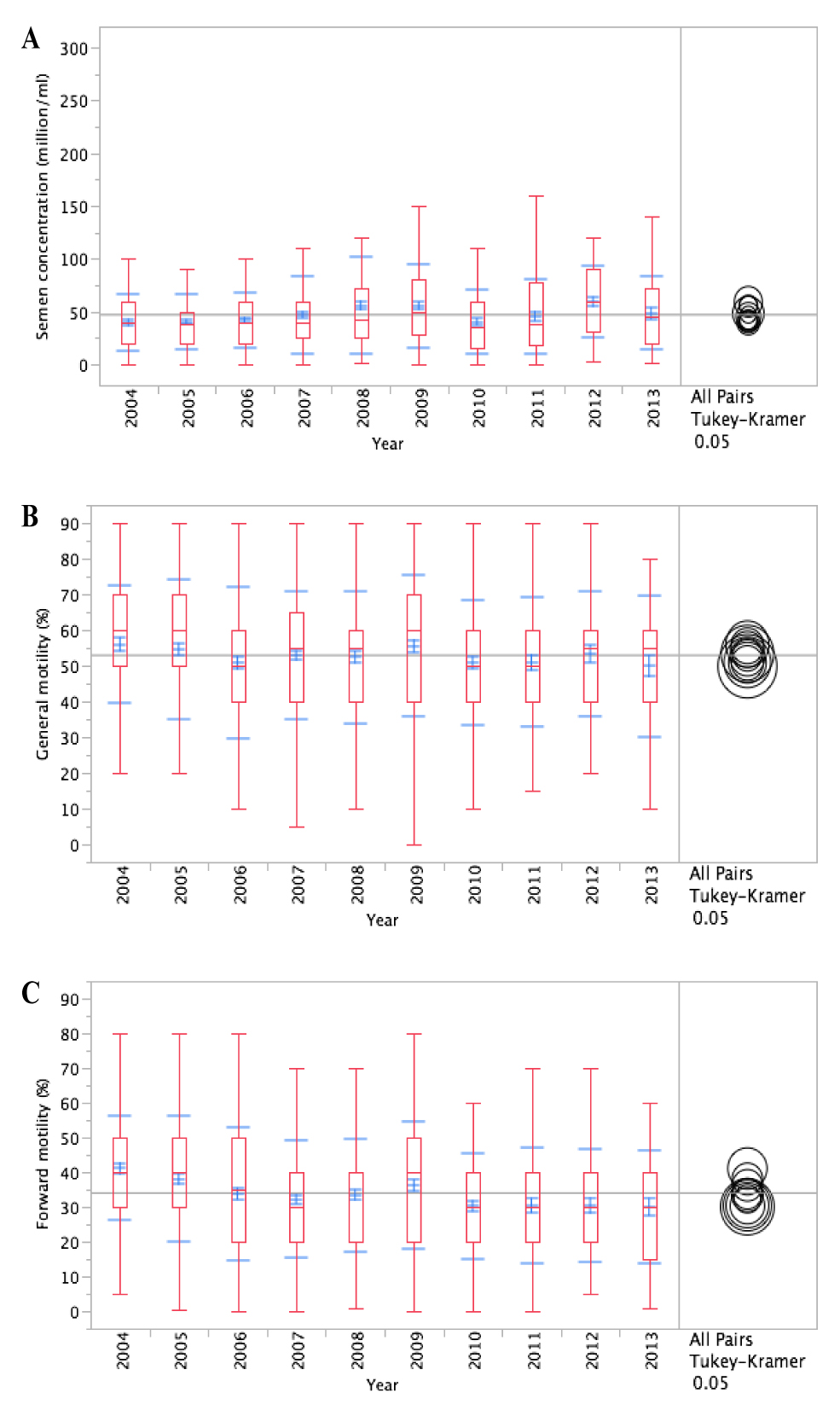
Figure 1. Box and Whiskers plot diagram of the continuous variables of interest, of the semen quality parameters by year from 2004 to 2013. For each year the diagram represents: red whisker (minimum and maximum value), blue whisker (mean ± SD), red box (mean; 5th and 95th percentile). A. Sperm concentration (million/ml); B. Sperm motility (%); C. Rapid progressive motile sperm (%).
The mean percentage of motile sperm (WHO a-c) during the study period was 52.9% (SD: 19%) with the highest value found in 2004 (56%, SD: 16.5%) and the lowest value found in 2013 (49.8%, SD: 19.9%). The overall difference between the two values was not statistically important (56% versus 49.8%; p=0.239), as well as the rest of the values, despite a sense of gradual deterioration (Figure 1). In addition, local polynomial smoothing, used to compare data with the mean of the variable, failed to reveal any kind of temporal trends of sperm motility within the decade of the study (Figure 2).

Figure 2. Scatterplot diagrams depicting individual values of the continuous variables of interest, of the semen quality parameters by year from 2004 to 2013. Local polynomial smoothing was used in order to assess visually temporal trends in the data (green line). Results are compared with the horizontal line (black line) that is defined by the mean value of the studied variable. A. Sperm concentration (million/ml)- No clear temporal trend; B. Sperm motility (%)- No clear temporal trend; C. Rapid progressive motile sperm (%)-clear downward temporal trend.
The mean percentage of rapid progressive motile sperm (WHO a) during the study period was 34.1% (SD: 17.4%) with the highest value found in 2004 (41.1%, SD: 15%) and the lowest value found in 2013 (30%, SD 16.2%). The overall difference between the two values was statistically significant (41.1% versus 30%; p<0.001) (Figure 1). Local polynomial smoothing, used to compare data with the mean of the variable, revealed a temporal downward trend of the progressively rapid motile sperm within the decade of the study (Figure 2).
The percentage of oligospermic males within the population of the study, presenting with a sperm concentration of less than 20 million/mL9 showed wide annual variations during the ten years of the study period. The highest value was found in 2001 (27.8%) and the lowest in 2009 (16.9%). At the beginning of the study in 2004, the percentage of oligospermic males was 23.5% revealing a slight improvement until 2009 (16.9%). From that point on, the annual percentage exhibits great fluctuations. When the Pearson Chi-squared test and the Chi-squared test for trend were employed, in order to test for the independence and for the trend of the percentage of sperm concentrations respectively, 20 million/ml and the year of study, no relationship was found (p=0.249) (Figure 3).
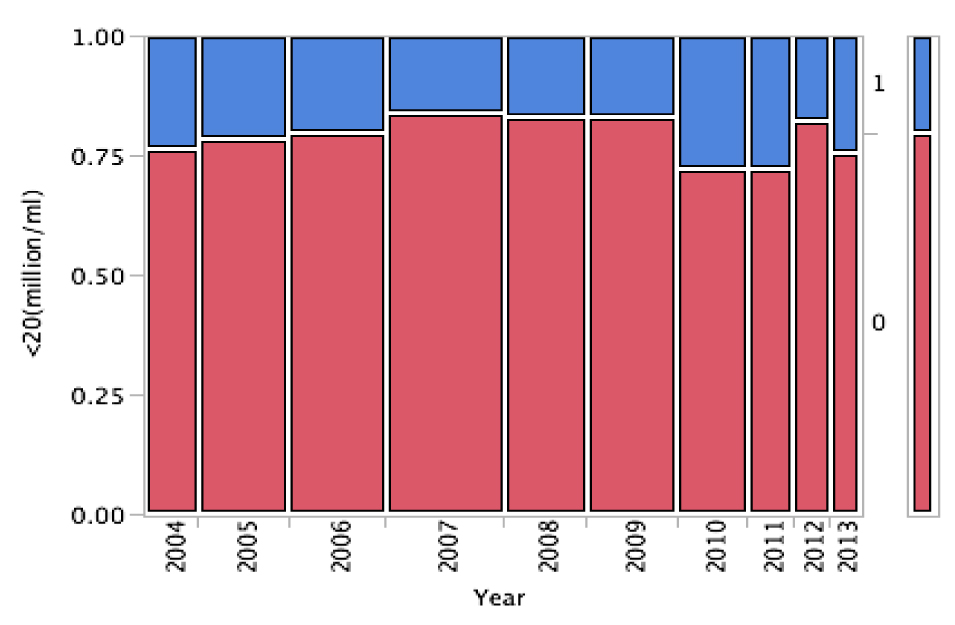
Figure 3. Contingency mosaic plot diagram between the annual percentage of sperm concentration > 20 million/ml (0/red) and the annual percentage of sperm concentration < 20 million/ml (1/blue) from 2004 to 2013.
Discussion
This retrospective observational study provides evidence that the sperm concentration of 1113 male partners of infertile couples seeking oocyte donation treatment in a single IVF center in Thessaloniki, Greece significantly increases over a period of ten years but at the moment, it does not describe a temporal upward trend. In addition, sperm motility, as well as the annual percentage of oligospermic males presenting with a sperm concentration of less than 20 million/mL remain relatively stable over time, without any statistically significant changes and with an absence of temporal trend. In contrast, rapid progressive sperm motility appears to deteriorate significantly, revealing a downward temporal trend over a period of ten years.
Semen parameters of male partners of infertile Greek couples were evaluated in an earlier retrospective study revealing a statistically significant reduction in sperm count over a period of 17 years. The survey included a total of 2385 native Greek men living in the greater area of Athens, randomly selected from a pool of 23850 couples of unknown fertility, attending three different andrological laboratories during a sub-fertility assessment8. These results were observed in concert with a parallel progressive incline of the environmental pollutants of the urban area over time, implying a causational relationship. Some may argue that selection and methodological bias was not avoided as population recruitment and semen analysis was performed by different technicians in three different laboratories with different number of examinations of sub-fertile couples. Our results did not confirm the aforementioned conclusions as we failed to reveal any kind of temporal trend with respect to sperm concentration and motility with the exception of rapid progressive motile sperm which exhibited a time-related deterioration. In fact, instead of a constant decline in sperm concentration, we observed a significant increase during the study period which was more profound in certain periods but yet fragmented and not clear enough in order to form a visible upward trend. Sperm motility and annual percentage of sperm concentration< 20 million/ml remained stable. Our results do not exclude that such a decline in sperm concentration may have occurred in the past in the male population of Athens or even more in the male population of Thessaloniki as well, but it is not evident in the present study. In addition, this discrepancy may be justified as the time frame of the two studies and the population recruited are quite different.
Our primary results seem to be in agreement with two recently published prospective studies. The first one addressed the matter of a possible secular trend among young Swedish men from the general population over the last decade. They concluded that there was no evidence of a time-related decrease in semen parameters in terms of sperm concentration (78 million/ml versus 82 million/ml; p=0.54), semen volume (3.1 ml versus 3.0 ml; p=0.26), sperm count (220 million/ml versus 250 million/ml) and progressive sperm motility11. The second one addressed the same question among young Danish men from the general population over a period of 15 years and concluded that there was an increasing trend in sperm concentration (43 million/ml versus 48 million/ml; p=0.02) and sperm count (132 million/ml versus 151 million/ml; p=0.001) even though only 25% had optimal sperm quality12. On the other hand, the results of the present study are contrary to the conclusions drawn from a large-scale multi-center retrospective study conducted in France over a period of 17 years, evaluating the semen quality in male partners of totally infertile women. The authors argued that there was a significant and continuous decrease in sperm concentration of about 1.9% per year, a significant decrease in normal sperm morphology, but without any temporal trend in sperm motility13.
Since the release of the Carlsen study, several major studies have been published evaluating temporal trends of semen parameters of males either of unproven fertility (general population) or of known fertility status (fertile or infertile). While only 8 of them revealed a decline in semen values, 21 suggested either no change or an occasional increase, and the other 6 showed conflicting results4,5. These discrepancies are mainly attributed to the intrinsic flows of the studies as most of them are retrospective, containing limited participants, who are highly heterogeneous and not properly adjusted for confounding factors. In addition, semen quality values demonstrate both geographical and temporal (seasonal) variation14, as well as they are being greatly influenced by a variety of factors such as intra and inter-laboratory variations, age, time of sexual abstinence, frequency of ejaculation, lifestyle habits, environmental pollutants, education, socioeconomic status5,15,16.
While declining sperm count is still controversial, it has been suggested by many authors that increased exposure, especially in fetal and pre-pubertal life, to endocrine-disrupting chemicals can be a possible causative mechanism of reduced sperm production. In fact, it is suggested that regional differences between rural and urban areas observed in semen quality can partially be attributed to exposure to different levels of environmental pollutants17. It seems likely that genetic inheritance and environmental factors interplay, defining reproductive function in two ways. Firstly, genetic predisposition can modulate the susceptibility of spermatogenesis and spermatozoa to the adverse effects of endocrine-disrupting compounds. Secondly, environmental factors can cause epigenetic modifications (DNA methylation, histone modification) with a major impact on sperm DNA integrity18.
The observed variation of semen attributes described in the literature doesnot seem to increase the time to pregnancy or cause impairment of men’s fertility19. Even though it has been shown that fertile partners of pregnant women exhibit decreasing trends of sperm concentration over time, the normal lower value provided in the manual of WHO in 1999 is 20 million/ml. In addition, population fecundity appears to either increase or at least remain stable over the last decades probably because of more efficient coital acts or reduction of sexually transmitted diseases20,21. Threshold values of sperm concentrations are of subjective importance in clinical practice because oligospermic men may still have a chance of conception and in reverse, normospermic men can be infertile22. Several authors have argued that when sperm concentration is below 40 million/ml the fecundity decreases and the time to pregnancy increases19,23,24.
The major strength of the present study was the highly homogeneous and well-defined population recruited over the extended period of ten years, which was ethically and regionally homogeneous as they shared the same environmental conditions. Data were collected from a single fertility center subjected to internal quality control, and analysis of the semen parameters was carried out by the same highly qualified biologist over the entire period, using standardized laboratory procedures and equipment, according to WHO-published manuals (WHO, 2009). On the other hand, the population studied was not adjusted for several confounding factors due to the partial lack of relevant information in the register. Also, the sample size exhibited annual variations and the participants were of advanced age, therefore a degree of selection bias cannot be excluded. Large-scale, population-based studies are needed to confirm our results.
Conclusions
The present retrospective study ends a long-lasting paucity of data as it consists the second report ever, evaluating semen parameters among the Greek population. It provides evidence that sperm concentration of male partners of infertile couples seeking oocyte donation treatment significantly increases over a period of ten years, but at the moment it does not form a visible temporal upward trend. In addition, sperm motility, as well as the annual percentage of oligospermic males presenting with a sperm concentration of less than 20 million/mL remain relatively stable over time, without any statistically significant changes and with an absence of temporal trend. In contrast, rapid progressive sperm motility appears to deteriorate significantly, revealing a visible time-related downward trend over a period of ten years. There is certainly a need for prospective, population-based, large-scale studies among the Greek population in an attempt to provide sound evidence in terms of possible temporal trends of semen quality parameters.
Disclosure of conflicts of interest
The authors report no conflicts of interest
Funding
No funding
Acknowledgments
None
References
- Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology. 2011;22:617-9.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609-13.
- Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod. 1997;12:2701-5.
- Fisch H. Declining worldwide sperm counts: disproving a myth. Urol Clin North Am. 2008;35:137-46, vii.
- Jouannet P, Wang C, Eustache F, Kold-Jensen T, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS. 2001;109:333-44.
- Neuwinger J, Behre HM, Nieschlag E. External quality control in the andrology laboratory: an experimental multicenter trial. Fertil Steril. 1990;54:308-14.
- Farrow S. Falling sperm quality: fact or fiction? BMJ. 1994;309:1-2.
- Adamopoulos DA, Pappa A, Nicopoulou S, et al. Seminal volume and total sperm number trends in men attending subfertility clinics in the greater Athens area during the period 1977-1993. Hum Reprod. 1996;11:1936-41.
- World Health Organization: WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction Cambridge: Cambridge University Press, 1999. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction Cambridge 1999;4.
- Makler A. The improved ten-micrometer chamber for rapid sperm count and motility evaluation. Fertil Steril. 1980;33:337-8.
- Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod. 2011;26:1012-6.
- Jorgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2.
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462-70.
- Jorgensen N, Andersen AG, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012-9.
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231-45.
- Lerchl A, Nieschlag E. Decreasing sperm counts? A critical (re)view. Exp Clin Endocrinol Diabetes. 1996;104:301-7.
- Swan SH, Brazil C, Drobnis EZ, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414-20.
- Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287-98.
- Bonde JP, Kold Jensen T, Brixen Larsen S, et al. Year of birth and sperm count in 10 Danish occupational studies. Scand J Work Environ Health. 1998;24:407-13.
- Scheike TH, Rylander L, Carstensen L, et al. Time trends in human fecundability in Sweden. Epidemiology. 2008;19:191-6.
- Oakley L, Doyle P, Maconochie N. Lifetime prevalence of infertility and infertility treatment in the UK: results from a population-based survey of reproduction. Hum Reprod. 2008;23:447-50.
- Jarow JP, Sharlip ID, Belker AM, et al. Best practice policies for male infertility. J Urol. 2002;167:2138-44.
- te Velde ER, Bonde JP. Misconceptions about falling sperm counts and fertility in Europe. Asian J Androl. 2013;15:195-8.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Role of semen analysis in subfertile couples. Fertil Steril. 2011;95:1013-9.