Research
HJOG 2024, 23 (1), 59-68| doi: 10.33574/hjog.0550
Noha Abd El-Sattar Afify Sakna, Mohamed Hassan Nasr El Din, Mourad Moustafa Attia Mohamed, Ahmed Mohamed Zeinhom
Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Correspondence: Noha Abd El-Sattar Afify Sakna, Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt, Tel.: 01009876789, E-mail: Noha@yahoo.com
Abstract
Introduction and the Αim: 2D and 3D biometry measures can record the increased development of the fetal brain in the later part of gestation. The objective of this study is to determine whether or not the frontal brain regions of fetuses with congenital heart disease (CHD) are smaller than those of healthy controls.
Patients and Μethods: The fetal Medicine unit of the Ain Shams University Maternity Hospital carried out a comparative cross-sectional study, including 140 normal fetuses and 140 fetuses with isolated CHD evaluated between 20 and 39 gestational weeks at our fetal medicine unit in the period November 2021–September 2023
Results: Our results showed the occipito-frontal diameter (OFD) and frontal antero-posterior diameter (FAPD) utilising ultrasonography. FAPD Mean±SD: 26.53 ± 10.44, OFD Mean±SD: 89.14 ± 28.34, and FAPD/OFD Mean±SD: 0.29 ± 0.05. The OFD Mean±SD was found to be 82.18 ± 18.42, the FAPD Mean±SD to be 31.68±6.58, and the FAPD/OFD Mean±SD to be 38.70±2.15. Our results showed that 0.7% of patients exhibited anomalies related to the central nervous system, such as dilated posterior fossa, vermis = 25 mm, and aberrant posterior cranial fossa. The percentage of those with a history of CHD was 3.9%.The history of CHD showed a statistically significant difference between the research groups. When examining comorbidities, surgical history, and the relationship between the other parameters and FAPD, OFD, and FAPD/OFD, there was no statistically significant difference between the groups under investigation, except for CNS abnormalities and gestational age.
Conclusion, compared to normal fetuses, fetuses with CHD had shorter FAPDs and a lower FAPD/OFD ratio. Despite the fact that different types of CHD affect hemodynamics differently, there appears to be a common deficiency in the development of the frontal cortex.
Keywords: Fetuses, congenital cardiac disease, frontal lobe, and ultrasound parameters
Introduction
Neonates with congenital heart disease (CHD), especially those with a univentricular heart architecture, show evidence of delayed brain development and neurodevelopmental delay (NDD) prior to surgery. 1 Lately, it has been demonstrated that fetal CHD has uneven cerebral blood flow, delayed sulcation, and a smaller developing brain.2 This seems to be the consequence of changed hemodynamics, which suggests a reduced supply of oxygen to the brain, especially in fetuses with univentricular cardiac circulation.3 It’s interesting to note that preliminary research on fetuses suggests that these biometric changes may be detected as early as the second trimester of pregnancy.
Furthermore, data suggests that the abnormal hemodynamics in newborns suffering from catastrophic CHD impact different parts of the brain, with the development of the frontal lobe being more severely disrupted, especially in cases where the heart architecture is univentricular.4 It looks that early fetal research had similar results.5 However, the method employed—which depended on a threedimensional (3D) reconstruction of the fetal brain volume—was relatively intricate, and there aren’t many data available.6 The objective of our study is to determine whether or not the frontal brain regions of fetuses with congenital heart disease (CHD) are smaller than those of healthy controls.
Patients and Methods
The fetal Medicine unit of the Ain Shams University Maternity Hospital carried out a comparative cross-sectional study, including 140 normal fetuses and 140 fetuses with isolated CHD evaluated between 20 and 39 gestational weeks at our fetal medicine unit in the period November 2021–September 2023. All sonars were done by 4 specialists using the same protocols of measurements to avoid interindividual variability and increase reliability.
Two cohorts of singleton pregnant women were selected for prenatal treatment from the Ain Shams University Maternity Hospital’s outpatient obstetrics clinic: Study Group: Women with no extracardiac anomalies on fetal echocardiography who were diagnosed with fetal cardiomyopathy; Women in the control group had normal pregnancy and newborn outcomes, no significant cardiac or extracardiac abnormalities, and no restrictions on the development of the fetus.
The study group (fetal echocardiography-based antenatal diagnosis of congenital heart disease and lack of extracardiac abnormalities) and the control group (no fetal growth restriction and a normal fetus without significant cardiac or extracardiac abnormalities) met the inclusion criteria.
To avoid misconceptions during the follow-up period, exclusion criteria include the following: the presence of twin pregnancy; limitations on fetal development to rule out syndromatic abnormalities; and associated extracardiac deformities to rule out syndromatic anomalies.
The following tests were performed on each patient: Following a comprehensive clinical assessment that included an ultrasound examination, a detailed history, a general examination, an abdominal examination, and investigations, each research subject provided their informed consent.
Sample Size Justification:
It is anticipated that 140 women per group will be required to detect a difference between the two groups using the PASS 11 application to compute sample size. Metrics for results: The principal result in fetuses with congenital heart disease (CHD) is affection of the frontal lobe; other brain areas follow later to the basic outcome.
Ethical Considerations:
The ethics committee of the department of obstetrics and gynaecology at Ain Shams University’s college of medicine authorised the study that would be presented. After informing the subjects about the goal and procedures of the study, volunteers gave their informed permission. The diagnosis will be used to convey the data, not the patient’s name.
Statistical methods: The collected data was coded, tabulated, and statistically analysed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013.
P <0.05 was used to determine statistical significance. All analyses were performed using Stata for Windows 13.1 (Stata Corporation, College Station, TX, USA). Propensity score analysis was performed using the p score and attnd tools that Becker and Ichino implemented in Stata. (Ichino and Becker, 2002).

Figure 1. It shows the fetal head’s transventricular axial plane at 21 week gestational age.
Results
We evaluated 140 normal controls and 140 cases with isolated CHD, between 20 and 39 gestational weeks. Demographic data for each group are given in Table 1. There was no significant difference between the two groups regarding the age of women,Parity and gestational age. Table 2 showed a Comparison between normal and congenital heart disease regarding surgical history and co-morbidities. With the exception of diabetes and consanguinity, there was no statistically significant difference in the surgical history or co-morbidities between fetuses with normal heart disease and those with congenital heart disease. Regarding DM and consanguinity, there was a statistically significant difference between congenital heart disease and normal heart disease. Regarding DM, HTN, Cardiac, Consanguinity, and Other CNS abnormalities with Relation to OFD, there was no statistically significant difference. Regarding medications, heart illness, cardiac history, and cardiac conditions, there was a very statistically significant difference (Table 3).
The relationship between FAPD/OFD and other parameters, including surgical history, diabetes, heart disease, consanguinity, medications, other CNS abnormalities, and history of congestive heart failure, did not differ in a statistically significant way.With the exception of cardiac illness A statistically significant difference was seen in the relationship between cardiac heart disease and FAPD/OFD (Table 4). Table 5 and Figure 2 show Correlation of FAPD, OFD and FAPD/OFD with other studied parameters in all cases. Regarding the correlations between age, parity, and gestational age and FAPD, OFD, and FAPD/OFD, there was no statistically significant difference. Regarding other CNS defects, there was no statistically significant difference between congenital heart disease and normal heart disease. When it came to the history of congenital cardiac disease, there was a statistically significant difference between the two conditions (Table 6).
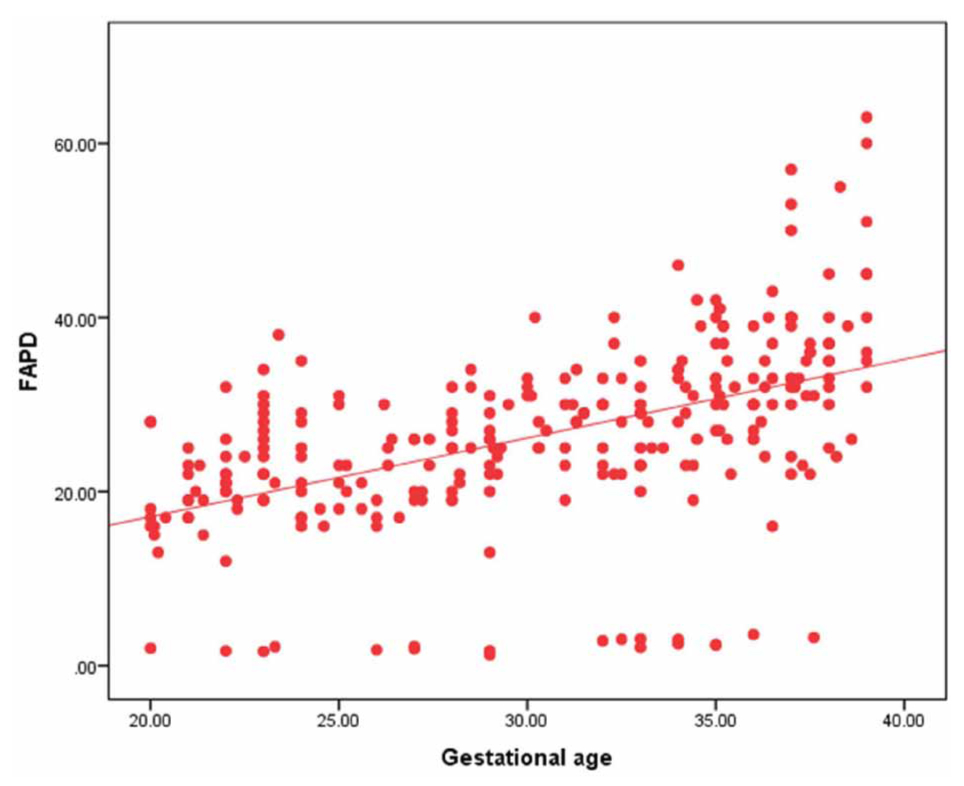
Figure 2: Correlation of FAPD with gestational age in all cases.
There was no significant difference between the two groups regarding the age of women, Parity and gestational age.
With the exception of diabetes and consanguinity, there was no statistically significant difference in the surgical history or co-morbidities between fetuses with normal heart disease and those with congenital heart disease.
Regarding DM and consanguinity, there was a statistically significant difference between congenital heart disease and normal heart disease.
Regarding DM, HTN, Cardiac, Consanguinity, and Other CNS abnormalities with Relation to OFD, there was no statistically significant difference. Regarding medications, heart illness, cardiac history, and cardiac conditions, there was a very statistically significant difference.
The relationship between FAPD/OFD and other parameters, including surgical history, diabetes, heart disease, consanguinity, medications, other CNS abnormalities, and history of congestive heart failure, did not differ in a statistically significant way.
With the exception of cardiac illness A statistically significant difference was seen in the relationship between cardiac heart disease and FAPD/OFD.
Regarding the correlations between age, parity, and gestational age and FAPD, OFD, and FAPD/OFD, there was no statistically significant difference.
Regarding other CNS defects, there was no statistically significant difference between congenital heart disease and normal heart disease. When it came to the history of congenital cardiac disease, there was a statistically significant difference between the two conditions.
Discussion
Infants with congenital heart disease (CHD), especially those with a univentricular heart architecture, show neurodevelopmental delay (NDD) symptoms prior to surgery. 2 Congenital heart disease (CHD) fetuses have uneven cerebral blood flow, delayed sulcation, and a smaller developed brain. These findings are supported by recent studies. This appears to be related to altered hemodynamics, especially in fetuses with univentricular cardiac circulation, which explains why the brain receives less oxygen. Notably, early studies in the foetus appear to show that these biometric alterations can be identified as early as the second trimester of pregnancy. It has also been shown more recently that the aberrant hemodynamics in neonates do not impact all areas of the brain in the same way, with the frontal cortex experiencing a more marked delay in brain growth and maturation. This effect in the foetus has also been shown in preliminary studies 3.
The results are consistent with those of Paladini et al., who found that there was a mean difference of 33.2 ±4.1, 28.3 ±5.4, and 37 ±2 weeks between the maternal age (years) and the GA at diagnosis (weeks) 7.
The current study included co-morbidities such as heart disease (0.7%), diabetes (18.2%), hypertension (11.4%), and surgical history (53.6%). Consanguinity was 16.1%. There were 50.0% of cases of congenital heart illness. Thirty percent of people used drugs. 13.9% of insulin Aldomet was 6.8%.
The purpose of the study by Al-Fahham et al. was to ascertain the role of fetal echocardiography in the early identification of fetal cardiac anomalies. They found that perinatal factors for congenital heart disease (CHD) were present in 65.3% of the pregnant mothers who were referred; consanguinity was detected in 31.7% of the cases; medication use was documented in 4.9% of the cases; and maternal illness was present in 22.8% of the cases (diabetes in 10.9%, lupus in 6.9%, and hypertension in 4.8%).
Our results showed the occipito-frontal diameter (OFD) and frontal antero-posterior diameter (FAPD) utilising ultrasonography. FAPD Mean±SD: 26.53 ± 10.44, OFD Mean±SD: 89.14 ± 28.34, and FAPD/OFD Mean±SD: 0.29 ± 0.05.
The OFD Mean±SD was found to be 82.18± 18.42, the FAPD Mean±SD to be 31.68±6.58, and the FAPD/OFD Mean±SD to be 38.70±2.15, according to Paladini et al. These results are consistent with our observations.
Our results showed that 0.7% of patients exhibited anomalies related to the central nervous system, such as dilated posterior fossa, vermis = 25 mm, and aberrant posterior cranial fossa. The percentage of those with a history of CHD was 3.9%.
Babies and fetuses with congenital heart disease (CHD) show lower brain sizes, delayed brain development, and nonverbal dementia, especially in those with univentricular cardiac architecture.It is noteworthy that preliminary research on the fetus seems to indicate that these biometric changes can be detected as early as the second trimester of pregnancy 6.
Recent research has also demonstrated that the abnormal hemodynamics in newborns affect different parts of the brain, with the frontal cortex showing a more pronounced delay in brain development 2. Preliminary study has also demonstrated this behaviour in fetuses. The method used to assess the preferential limitation of frontal lobe growth due to the fetal brain volume was a bit complex because it used 3D reconstruction 8.
The current study identified no statistically significant variations in surgical history or co-morbidities between individuals with and without congenital cardiac disease, with the exception of DM and consanguinity. There was a statistically significant difference between congenital heart disease and normal heart disease with regard to DM and consanguinity.
Our results showed that there was no statistically significant difference between congenital heart disease and normal heart disease in terms of other CNS abnormalities. There was a statistically significant difference in the history of congenital heart disease compared to normal heart disease.
Our data were in accord with the results of ZENG et al. (adjusted R2=0.916 for total intracranial volume, 0.796 for frontal lobe volume, 0.864 for thalamus volume, and 0.852 for cerebellar volume). They found that in fetuses with congenital heart disease (CHD), the diagnostic category (P<0.001) was independently associated with decreased brain sizes; the biggest differences were observed in cases of HLHS, which were followed by aortic hypoplasia, TGA, and TOF (P<0.001). Between the patients and controls, there was a significant difference in each structure and total intracranial volume. 9 Our results showed that there was no statistically significant association between FAPD, OFD, and FAPD/OFD and age, parity, and gestational age.
Our research showed no statistically significant difference in the association between FAPD and prior surgical experience, diabetes, hypertension, and other CNS disorders. There was a statistically significant difference in the association between FAPD and maternal cardiac disease and history of CHD.
Our results were consistent with PENG and Ruan’s, who found no significant difference in the distribution of FAPD/OFD ratios between fetuses with FGR and those without FCR (37.561.16 and 37.201.40, respectively; P=0.383). There was no significant difference seen in the FAPD/HC of fetuses with FGR and those without FCR (11.92±0.63 and 11.77±0.72, respectively; P=0.410). 10
In the current study, there was a statistically significant difference in the connection between gestational age and FAPD, OFD, and FAPD/OFD. There were statistically significant differences in the correlations between the OFD and gestational age, age, and parity. There was no statistically significant difference in the connection between age and parity, FAPD, and FAPD/OFD respectively.
The groups with left-heart lesion or univentricular heart and all other CHDs had significantly lower FAPD/OFD ratios compared to normal fetuses (P < 0.0001). There was no statistically significant difference seen between the two CHD groups. The association between FAPD and the history of CHD, DM, HTN, heart, consanguinity medications, and surgical history was not observed to vary significantly in the current study.
Trends in the FAPD and FAPD/OFD ratio can be used to identify changes in the fetal frontal lobe during pregnancy. Our results show that while the FAPD/OFD ratio is generally steady, it did show some slight fluctuations during fetal development. These changes suggest that different regions of the brain grow at different rates. Myelination begins in the third or sixth month of fetal development. The next regions that get myelination are the internal capsule, occipital, splenic, parietal, temporal, frontal, and genu white matter areas. 11,12
Conclusion
Fetuses with congenital heart disease (CHD) had shorter FAPDs and a lower FAPD/OFD ratio than normal fetuses. All types of congestive heart failure seem to have this delayed growth of the brain’s frontal lobe, regardless of the impact on hemodynamics.
Strengths and weaknesses of the study
The comparatively high number of cases and controls, together with the straightforward approach that can be readily applied to saved photos, are two of the study’s strengths. The very limited number of cases in each of the CHD categories is a restriction that made it impossible to conduct a thorough study by type of CHD. Our use of a two-dimensional technique, presuming that the maximal cross-section of the frontal lobes corresponds to the cerebral region delineated by the frontal and parietal bones laterally and a line crossing tangential to the posterior boundary of the CSP, is another drawback. Nevertheless, we believe that this straightforward technology is adequate because it may indicate a considerable regional deficit in brain development.
Summary-significance of the study
Foetuses with congenital heart disease (CHD) have decreased brain development in the frontal lobes. Regardless of how the various forms of CHD affect hemodynamics, this appears to happen in all of them.
This study demonstrates that the foetal frontal cortex’s poor development is linked to the abnormal hemodynamics brought on by congenital heart disease. To determine if the decreased growth is linked to anomalies in this area, more research is required to match these results with assessments of frontal cortical maturation.
No particular grant from a governmental, private, or nonprofit organization was given for this research.
No conflict of interests
Author contribution
Each author came up with the concept, created the theory, carried out the calculations, and examined the analytical techniques. Each author contributed to the final text and discussed the findings. Everyone completed the experiment and penned the paper.
Referances
- Masoller N, Sanz-CortéS M, Crispi F, Gmez O, Bennasar M, Egaña-Ugrinovic G, Bargallό N, Martínez JM, Gratacόs E. Mid-gestation brain Doppler and head biometry in fetuses with congenital heart disease predict abnormal brain development at birth. Ultrasound Obstet Gynecol 2016; 47: 65–73.
- Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, Xu D, McQuillen P. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg 2018; 155: 291–300.e3.
- Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, Hickey E, Miller S, Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015; 131: 1313–1323.
- Ortinau C, Beca J, Lambeth J, Ferdman B, Alexopoulos D, Shimony JS, Wallendorf M, Neil J, Inder T. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg 2012; 143: 1264–1270.
- Peng Q, Zeng S, Zhou Q, Deng W, Wang T, Tan Y, Liu Y. Different vasodilatation characteristics among the main cerebral arteries in fetuses with congenital heart defects. Sci Rep 2018; 8: 4544.
- Zeng S, Zhou QC, Zhou JW, Li M, Long C, Peng QH. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet Gynecol 2015; 46: 174–181.
- Paladini D, Finarelli A, Donarini G, Parodi S, Lombardo V, Tuo G, Birnbaum R. Frontal lobe growth is impaired in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2021 May;57(5):776-782.
- Khalil A, Bennet S, Thilaganathan B, Paladini D, Griffiths P, Carvalho JS. Prevalence of prenatal brain abnormalities in fetuses with congenital heart disease: a systematic review. Ultrasound Obstet Gynecol2016;48: 296 – 30
- Zeng, S., et al. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound in Obstetrics & Gynecology, 2015, 46.2: 174-181.
- Peng, Ruan, et al. Frontal lobe development in fetuses with growth restriction by using ultrasound: a case–control study. BMC Pregnancy and Childbirth, 2022, 22.1: 1-10.
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic reso nance imaging. J Neurosci. 2011; 31:784–91
- Al-Fahham MM, Gad NA, Ramy ARM, Habeeb NM. Clinical utility of fetal echocardiography: an Egyptian center experience. Egypt Heart J. 2021 Aug 19;73(1):71. doi: 10.1186/s43044-021-00196-z. PMID: 34410524; PMCID: PMC8377121.