Review
HJOG 2025, 24 (4), 267-280| doi: 10.33574/hjog.0607
Stathakopoulou Nikolitsa, Kalampokas Emmanouil, Triantafyllidou Olga, Mastorakos Georgios, Vlahos Nikolaos, Kalampokas Theodoros
Department of Obstetrics and Gynaecology, Aretaieio Hospital, Athens, Greece
Correspondence: Stathakopoulou Nikolitsa, Contact telephone: +306943954053, E-mail: nicolastatha@gmail.com
Abstract
Introduction: Substance use during pregnancy presents critical risks to both maternal and fetal health. Addressing addiction in pregnant women requires a multidisciplinary and evidence-based approach.
Purpose: This review aims to explore treatment modalities for substance use disorders in pregnant women, with emphasis on clinical efficacy, regional programs, and best practices in Greece and Europe.
Methods: We conducted a literature review from PubMed, EMBASE, and Google Scholar using keywords such as “pregnancy,” “addiction,” “substance use disorder,” “methadone,” and “treatment” within the time frame 2000–2025. Fifty-two studies were included based on predefined inclusion and exclusion criteria.
Results: Methadone reduced illicit opioid use by up to 60% but increased neonatal abstinence syndrome by 30%. Buprenorphine showed fewer neonatal complications compared to methadone. Multidisciplinary programs improved maternal retention in care by 40%. Major barriers included social stigma, limited accessibility, and lack of integrated services. Greek-specific data highlighted gaps in coordination and underfunded regional initiatives.
Conclusions: Effective management of addiction in pregnancy necessitates integrated care models, updated clinical protocols, and targeted public health policies. Investment in prevention, staff training, and postnatal follow-up is crucial.
Keywords: Treatment, addictive substances, pregnant women, addicted women, interventions
Introduction
Addictive substances are natural or synthetic substances that affect the user’s Central Nervous System, causing addiction [1]. Addiction to psychotropic substances is an uncontrolled state in which users cannot resist their desire to use these substances, resulting in mental and physical depression accompanied by symptoms of physical pain, discomfort, and mental and social impoverishment [2]. These outcomes are often reinforced by neurological adaptations that alter reward circuits and impair decision-making [3].
Substance use during pregnancy is an escalating public health challenge, particularly in the context of the ongoing opioid epidemic and increasing cannabis legalization. Pregnant women with substance use disorders face elevated risks of obstetric complications, neonatal withdrawal syndromes, and long-term developmental impacts on the child. Despite its significance, therapeutic strategies specific to pregnant women remain under-researched, especially in regional contexts like Greece.The Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSMIV) distinguishes between substance dependence and substance abuse. Dependence refers to a pattern of compulsive use of a substance, often accompanied by tolerance and withdrawal symptoms, as well as serious psychological and physical difficulties. Substance abuse is a less serious disorder that is perceived as the user’s inability to meet their obligations and interpersonal and legal problems [4].
A similar reference is made by the International Statistical Classification of Diseases and Related Health Problems (ICD-10), where there is a distinction between harmful use and dependence syndrome. The term harmful use refers to “the use of psychoactive substances that causes physical (e.g. hepatitis after drug injection) or mental damage (e.g. depression after alcohol use) to the health of the individual” [5]. In contrast, the dependence syndrome is characterized by the individual’s strong desire to use substances, which is accompanied by signs of physiological disorder that, depending on the type of substance, may show symptoms of tremor, sweating, anxiety, agitation, depression, nausea and discomfort, myalgia, muscle twitching, lethargy, instability, distortion of perception or distortion of body image, etc. [6].
Legal and illegal substances
Substances that cause dependence include not only the use of narcotic substances that are considered “illegal” and are criminalized, but also other “legal substances” that are quite familiar to the population such as caffeine, nicotine, and alcohol, which are allowed to be used freely [7]. There is also an additional category that includes psychotropic drugs such as sedatives and hypnotics, which are mainly available for therapeutic purposes upon a doctor’s order. Despite their regulated use, prescription drugs can also lead to dependence, especially with prolonged or unsupervised use [8].
The category of illegal substances includes drugs that have a serious harmful effect on the individual, such as heroin, cocaine, crystal methamphetamine, anabolic steroids, cannabis, marijuana, LSD, and MDMA (ecstasy) [2]. These substances are associated with high levels of dependence, neurotoxicity, and increased risk for mental and physical deterioration [9].The legalization of addictive substances often depends on political, cultural, and social factors. For example, tobacco was considered illegal when it first appeared in England, but was later legalized and became a source of revenue for the state [10]. In modern times, similar policy transitions have occurred with cannabis, which has been decriminalized or legalized in various countries for medicinal and recreational use [11].
Legal substances include alcohol, caffeine, and tobacco, which are freely available in the market, as well as prescription drugs such as antidepressants, anxiolytics, and sedatives, which are administered under medical supervision [8]. However, concern remains regarding the real safety of these substances. For example, although alcohol is legal and socially accepted, it is one of the leading causes of preventable illness and death globally [12]. Similarly, tobacco use remains a major public health issue, being the single most important preventable cause of death worldwide, despite its legal status [7].
Methods
Search Strategy: A comprehensive literature search was conducted using PubMed, EMBASE, and Google Scholar databases. Keywords included combinations of the following terms: “pregnant,” “pregnancy,” “substance use disorder,” “addiction,” “methadone,” “buprenorphine,” “treatment,” and “intervention.” Boolean operators (AND, OR) were used to refine search results. The timeframe covered publications from January 2000 to March 2025. References of included articles were manually screened to identify additional relevant studies.
Inclusion Criteria: Articles were eligible if they (1) were peer-reviewed, (2) focused on pregnant women with substance use disorders, (3) evaluated treatment or care strategies, (4) were conducted in Europe or provided comparative insights relevant to the Greek context, and (5) were published in English.
Exclusion Criteria: Studies were excluded if they (1) involved animal models, (2) were case reports, (3) addressed non-pregnant populations only, or (4) lacked a therapeutic or clinical focus.
Data Extraction and Synthesis: Relevant information was extracted into a structured table summarizing study characteristics, sample sizes, substance types, treatment modalities, and key outcomes. A thematic synthesis was performed to identify patterns and group results into categories such as epidemiology, treatment effectiveness, barriers to care, comorbidities, and regional programs. Quality assessment was based on study design, sample size, and clarity of reported outcomes.
Results
Epidemiology of Substance Use in Pregnancy
Epidemiology of dependent individuals in Europe
Substance use during pregnancy varies across Europe, with increasing cannabis use especially during the first trimester. Data indicate that up to 7% of pregnant women in the U.S. report cannabis use, with first-trimester prevalence exceeding 12%. In Greece, although overall use remains lower, a significant portion of pregnant users engage in polysubstance use. Underreporting and stigma affect the reliability of prevalence estimates. A summary of key prevalence data is presented in Table 1.
It is estimated that around 30,000 pregnant women in Europe use opioids each year, and the number of pregnant women who experience problems with other drugs may be comparable. This is of concern, as drug use during pregnancy is associated with a variety of adverse effects on the pregnant woman, which often have adverse effects on the developing fetus and newborn infant [13].
A representative survey of women aged 12 to 44 years conducted in the USA by the National Survey on Drug Use and Health (NSDUH) during the period 2002-2017, demonstrates an increase in daily/almost daily use and the number of days of use, among all pregnant (and non-pregnant) women. In addition, use appears to be higher in the first trimester than in the second and third trimesters of pregnancy. Comparing the periods between 2002-2003 and 2016-2017, the adjusted prevalence of cannabis use increased from 3.4% to 7.0% among pregnant women, from 5.7% to 12.1% in the first trimester of pregnancy, from 0.6% to 2.5% in the second trimester, and from 0.5% to 2.5% in the third trimester. This increasing trend may be due to the approval of cannabis for medical purposes in several US states, e.g. for the treatment of nausea/vomiting, especially during the first trimester of pregnancy. However, clinicians responding to the recommendations of the American College of Obstetricians and Gynecologists recommend that pregnant women discontinue cannabis use [14].
Epidemiology of dependent individuals in Greece
In Greece, a total of 116 treatment facilities and 47 counseling centers for the treatment of substance addiction operated in 2018. The total number of people who received treatment services in 2018 was 12,311, of whom 8,975 (73%) were in substitution treatment, 2,420 (20%) in “dry” treatment programs, 647 (5%) received interventions within the prison, while 269 people (2%) attended a physical detoxification program [15].
Based on the aggregate data for the period 2010-2018, a total of 4,735 people received counseling services in 2018, half of whom were first-time clients and 1 in 4 progressed to the main treatment phase of a program. In general, the number of people seeking help from the services of the Counseling Centers operating within the framework of “dry” treatment programs, as well as the percentage of people completing the treatment program, tends to decrease. The trend of users choosing to proceed to the main phase of addiction treatment also appears to be decreasing [15].
Regarding alcohol use, a total of 3,090 visits were made to counseling and treatment services in Greece in 2018. The survey data is encouraging as it demonstrates a decrease in the percentage of the total number of individuals who entered a treatment program for alcohol addiction by 4.5 percentage compared to previous years. However, despite the overall decrease, there is a trend towards an increase in the percentage of individuals over 50 years of age who entered a treatment program and also a slight increase in the percentage of early discharge from treatment programs (2017: 7.6%, 2018: 8.1%) due to absence from counseling/treatment sessions, alcohol use and non-compliance with the treatment contract [15].
Therapeutic treatment of addicted pregnant women
Treatment Efficacy
Pharmacological substitution therapies such as methadone and buprenorphine are mainstays of opioid dependence treatment in pregnancy. Methadone is associated with improved maternal retention in care but a higher risk of neonatal abstinence syndrome (NAS). Buprenorphine offers reduced NAS incidence but may result in lower maternal adherence. Psychosocial interventions such as cognitive-behavioral therapy (CBT) and motivational interviewing reduce relapse risk and enhance maternal engagement. Table 2 compares treatment modalities and outcomes.
Barriers to Care
The use of addictive substances during pregnancy poses serious risks for the mother and child. For this reason, the supportive care provided to pregnant women during the prenatal, perinatal and postnatal periods is very important both for the smooth progression of the pregnancy and during the neonatal period.The literature reports a series of problems that hinder pregnant women’s access to specialized support and care services. These barriers could be divided into internal and external. Internal barriers include a general refusal of women to receive help or some form of treatment, feelings of shame and guilt, concerns about the protection of their privacy and manifestations of phobia about losing custody of their children. External barriers mainly concern logistical and financial obstacles, waiting lists, lack of flexibility in service opening hours, lack of sensitivity by staff regarding gender differences, etc. [16].
Other factors such as the pregnant woman’s partner who may be a substance abuser himself, psychiatric comorbidity, previous traumatic experiences such as sexual abuse and violence, marital status (married, divorced, single, etc.), level of education, lack of income, difficulty integrating into support structures, etc. avoid pregnant women to specialized support and care services[16].
Psychiatric Comorbidities
The World Health Organization (WHO) defines “co-morbidity or dual diagnosis” as the coexistence of a substance use disorder and another psychiatric disorder in the same individual [17]. Research suggests that psychiatric and personality disorders coexist with substance use disorders, but may also be pre-existing or exacerbate them [18].
The diagnosis of comorbidity is a very difficult task for health professionals. The usual clinical picture of substance dependence and disordered behavior is often confused with manifestations due to psychiatric disorders. The manifestation of symptoms differs little from those of psychiatric disorders, while the withdrawal syndrome or acute intoxication can mimic almost all the symptoms of these disorders [19]. Fridell’s research on the psychopathology of substance-dependent individuals confirms comorbidity in substance dependence and identifies three main groups of disorders: personality disorders (65-85%), depressive and anxiety states (30-50%) and psychoses (15%) [20]. Furthermore, another relevant study conducted on drug users in treatment found the prevalence of antisocial (23%), borderline (18%) and paranoid (10%) personality disorders [21].
Lauritzen et al. found that a high proportion of substance abusers in Norway had experienced serious family problems during childhood and adolescence. Research data indicate that 70% of the individuals had learning problems at school, 38% had been bullied, and 21% had received some form of psychiatric treatment during childhood and adolescence [22]. Similarly, Beutel found that female substance abusers with psychiatric comorbidity were often victims of sexual abuse [23].
Comorbidity is a significant obstacle to the diagnosis and therapeutic intervention of health professionals. In cases where mental health professionals detect parallel substance use during psychiatric treatment, patients are often referred to addiction treatment programs. However, these programs may redirect them back to psychiatric services, resulting in a cycle of exclusion and fragmentation of care [24]. This “ping-pong effect” frequently leads to a breakdown in continuity of treatment and deterioration in patient outcomes [25].Such individuals are often treated with suspicion by psychiatric services and may not be accepted into mental health programs. Similarly, substance users diagnosed with mental health disorders are sometimes excluded from addiction services due to their psychiatric comorbidity [26]. For instance, in some countries like Spain and Italy, psychiatric services still tend to exclude individuals with substance use disorders. In Greece, 54% of drug treatment programs reportedly exclude individuals diagnosed with a mental illness [27].This systemic separation between psychiatric and addiction services represents a barrier to integrated treatment, despite increasing evidence supporting the need for co-treatment models in dual diagnosis populations [26, 28].
Another study in Australia reported that nearly half of mothers who used drugs were diagnosed with depression. These mothers had more previous pregnancies and were more likely to have experienced domestic violence. Given that pregnant women who use drugs may have worse prenatal care and social outcomes for themselves and their children, researchers suggest that assessment is a means of diagnosing psychosocial disorders in order to facilitate early interventions [29].
Regional Programs and Policies
In Greece, specialized programs like those offered by OKANA and KETHEA provide structured support to pregnant women who use substances. However, program outcomes remain modest, with completion rates around 30% [30, 31]. International programs emphasize early engagement, continuity of care, and individualized treatment plans to improve maternal and neonatal outcomes [32, 33]. Table 3 compares Greek and international programs and their outcomes.
The treatment of pregnant women who use drugs is considered critically important during the antenatal, perinatal, and postnatal periods [34]. Pregnancy in women who use drugs or alcohol should be regarded as a potential high-risk pregnancy and assessed on an individual basis according to their specific needs. Treatment interventions extend beyond substitution therapies to include comprehensive services delivered by specialized multidisciplinary teams offering medical care, psychosocial support, and harm reduction [35].
Health professionals should approach pregnant substance users with empathy and provide them with the same level of care and attention as women who do not use substances [36]. Ideally, healthcare providers, including midwives, should receive specialized training in the assessment and management of substance use in pregnancy [37, 38]. Furthermore, cooperation between gynecological departments, substance use specialists, and community-based medical and social services is essential to optimize care continuity and outcomes [30, 39].
Another aspect that must be taken into account in the assessment of women who are drug users is the combined factors such as alcohol use, smoking and poor social conditions that make the course of treatment difficult. Usually, most pregnant women who use cocaine and/or heroin are also cigarette smokers [29]. Consequently, there are difficulties in the assessment and therapeutic intervention of pregnant women by the scientific team that is not satisfied with addressing the negative effects of the addictive substance but also the difficult social conditions and possible psychiatric comorbidity of these women.
The specialization of therapists combined with the definition of a clear and flexible treatment protocol is essential to address the challenges faced in caring for pregnant women who use substances. This approach facilitates effective communication and collaboration between the surrogacy unit, the prenatal care team, and the postnatal care team [40]. Recent research highlights that multidisciplinary teams with specialized training improve treatment adherence and maternal and neonatal outcomes [41, 42].
The primary goal of the multidisciplinary team is to ensure the pharmacological, social, and psychological stability of the pregnant woman. Engagement and commitment to treatment by pregnant women represent the first successful step toward continuous support and supervision throughout the prenatal period [37, 39].
Preventive multidisciplinary treatment programs, especially during prenatal care, that include medical, social and psychological support are likely to have a positive impact on the final outcome of pregnancy. Good coordination and communication between the parties involved is the basis for the correct and timely assessment of the risks and needs of the pregnant woman during the early stages of pregnancy and the preparation of a care and support plan for the pregnant woman and the infant [30]. The assessment should come from a team of specialists from various specialties (e.g., medical staff, midwives, psychologists, drug treatment services and social services [43].
An important part of a successful therapeutic intervention is to inform pregnant women about the potential risks of substance use to the fetus and newborn. Information is also considered important for issues that need to be addressed after the birth of the infant (e.g. the need for medical monitoring of the newborn), the continuation of postnatal assistance from the medical services involved and the possible involvement of social services. In particular, clarifications on the issue of the involvement of social services, whose main concern is to ensure the care provided and good living conditions for the newborn and the removal of the infant from the mother when this is deemed necessary for the safety of the child, are vital for addressing the mother’s fears and ensuring a balanced emotional state [30].
Special support programs in Greece
In Greece, the first therapeutic programs developed around the beginning of the 1980s involved programs in closed therapeutic communities, where users could stay for at least a year to complete the program. Subsequently, other more flexible day or night programs, outpatient-type programs, as well as other more specialized ones involving women (pregnant and imprisoned), adolescents and people with psychiatric comorbidities, were developed. The bodies providing treatment and support for addicted individuals that implement “dry” and comprehensive support programs are: OKANA and KETHEA and are implemented in the therapeutic support structures that they develop at a national level, 18 and above which operates within the framework of the Attica Psychiatric Hospital and is active in “dry” programs that aim to provide counseling and psychosocial support to the user and their families, and two other “dry” programs, “IANOS” and “ARGO” which are implemented at the Thessaloniki Psychiatric Hospital [31].
The integrated programs which are based on the Therapeutic Community model, are adapted according to the individual’s specific needs. They include intensive detoxification programs, counseling and psychosocial support for individuals and their families and usually last from a few months to two years. The pre-integration phase in the Therapeutic Community concerns the user’s first contact, which is implemented in the Counseling Centers where assessment, support and preparation are carried out, lasting approximately two months. The main phase of the program takes place in a residential or outpatient Therapeutic Community, lasts from 9 to 12 months and aims at the user’s physical and mental detoxification. The intervention is completed in the Social Reintegration Centers with support on an external basis for approximately one year, while at the same time treatment and support are offered in the user’s family environment by the Family Support Centers. At the same time, there are short-term addiction treatment programs (duration up to 18 months) which present a more flexible form of treatment (open structures) and concern drug users who remain integrated and need less intensive support on a daily basis [31].
It is worth noting that a permanent priority of all program implementation bodies both at the policy-making and intervention levels is prevention. For this reason, priority is given to the implementation of action programs in the school community. It is important that the action of Prevention Centers / bodies in the school community increased during the 2017-2018 school year, with main interventions in the prevention and promotion of the psychosocial health of children [31].
Within the framework of the above therapeutic programs for addiction and support for addicted individuals, individualized programs for pregnant addicted mothers are included. The programs for pregnant women provide medical and pharmaceutical care, psychosocial and counseling support both during pregnancy and after the birth of the child. The main objective of the support structures is for addicted women to develop relationships of trust with healthcare providers, to participate in comprehensive addiction programs and to receive care and support from multiple health services in order to initially ensure the safety of the pregnancy and subsequently the health of the mother and the infant [31].
Substitution Medication Protocols
WHO and EMCDDA guidelines recommend methadone as the first-line substitution therapy, with buprenorphine as an alternative. Clinical management includes maintaining opioid plasma levels, flexible dosing, and avoiding withdrawal during pregnancy. A flowchart illustrating ideal clinical decision-making is provided in Figure 1.The administration of substitution substances such as methadone, buprenorphine, etc. is recommended as a treatment during pregnancy as it involves fewer risks for the pregnant woman and the fetus compared to the continuation of substance use [37]. According to research, substitution treatments for the abuse of opiates and other addictive substances contribute positively to the smooth outcome of pregnancy. The improved results of therapeutic interventions with the administration of substitution substances are due to combined factors, such as the stabilization of use (avoiding cycles of intoxication and withdrawal), the facilitation of the pregnant woman’s access to prenatal and postnatal care and support services, the improvement of nutrition and the ease of access of the pregnant woman to social services [40, 44].
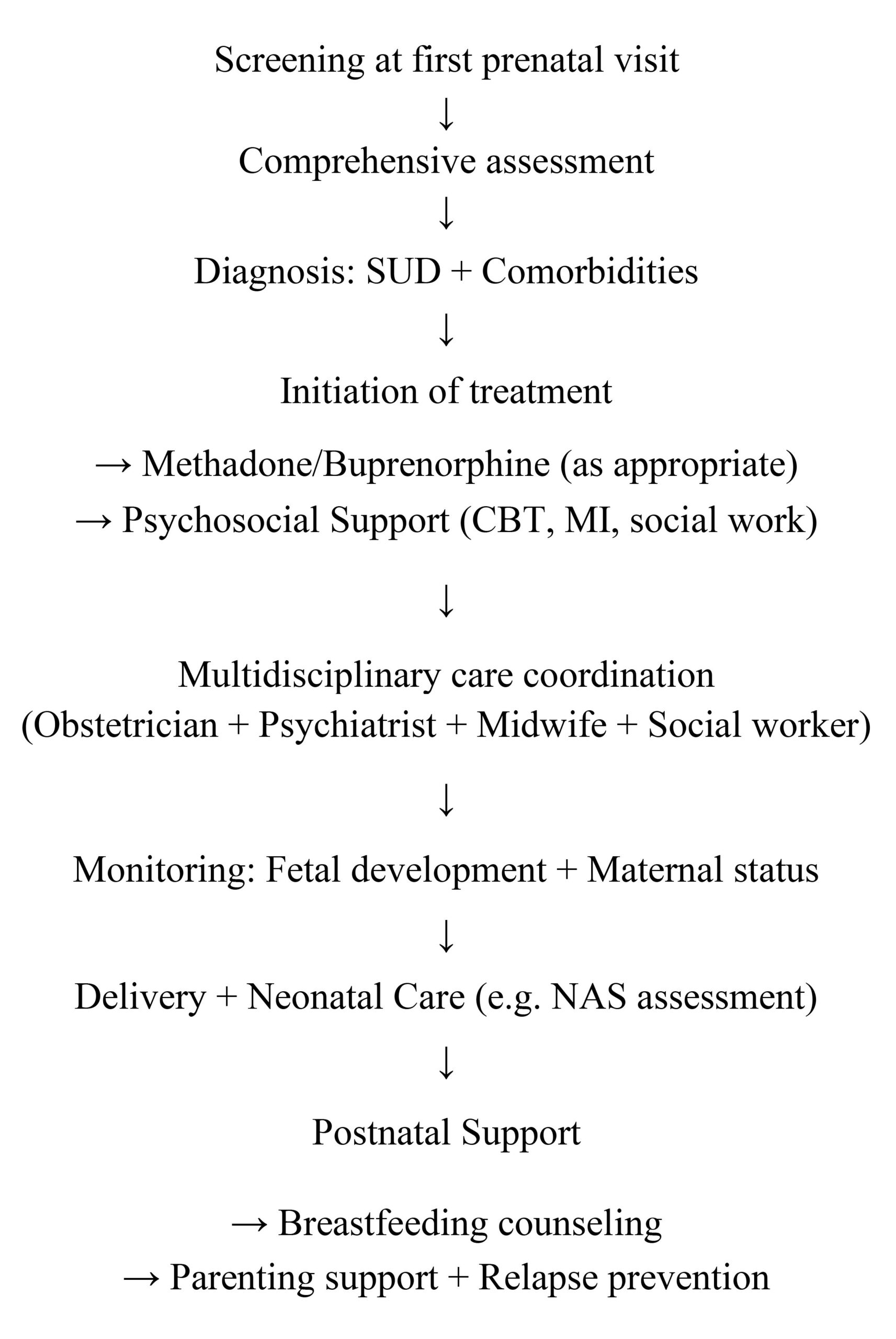
Figure 1. Ideal clinical pathway for the treatment of addicted pregnant women.
The most appropriate therapeutic approach for opioid substitution is ‘single dose maintenance’, which minimises continued use and can ensure good management of treatment during pregnancy and postpartum. In order to minimise risks, it is advisable to maintain stable blood levels of opioids in pregnant women. The dose administered should be adjusted according to the individual needs of the pregnant woman, which may vary throughout pregnancy and beyond the first days or weeks after delivery. For this reason, flexibility in the treatment plan is required in terms of the amount and frequency of dose reduction [40].
In Europe, the most common opioid substitution drugs are methadone, considered the most appropriate treatment, though it is associated to some extent with neonatal withdrawal syndrome (NAS) side effects. Buprenorphine is also used on a smaller scale, as slow-release oral morphine has not been sufficiently studied [44]. According to the World Health Organization (WHO) (2009), methadone is indicated due to its long history of safe use, although more recent studies suggest that buprenorphine administration may significantly reduce the incidence and severity of NAS in newborns [45].
A study reported that 28% of infants exposed to low methadone doses (5-30 mg) experienced NAS, increasing to 43% with moderate doses and 71% with high doses (51-95 mg) [46]. Despite these risks, the consensus remains that the benefits of opioid substitution therapy with methadone or buprenorphine during pregnancy outweigh the harms associated with continued opioid abuse by the pregnant woman [47].
Depending on the substances used, which may be more than one, specialist therapists determine the method of therapeutic intervention. For example, in women dependent on benzodiazepines, a diazepam stabilization program is initially followed and then a dose reduction program is applied. In pregnant women who are administered methadone, the dose should remain stable as an attempt is made to reduce the benzodiazepine. In women who are addicted to cocaine, it is recommended that they completely stop using it as there is no substitute substance, while psychological therapy is also provided. Similarly, support and treatment programs are implemented to limit or permanently stop smoking, with the aim of reducing the likelihood of low birth weight and premature birth, and alcohol use to limit neonatal alcohol syndrome (FAS) [30].
Breastfeeding and Postnatal Care
The safety and support of pregnant women who are addicted to substances does not stop with the safe completion of childbirth as the goal of the multidisciplinary team and all involved agencies and services is to maintain the care and support of the mother and child. In the event that the mother wishes to detoxify and raise her child, the support program should additionally include counseling, skills training and mental health advice. Furthermore, the possibility of withdrawal syndrome in newborns due to the administered methadone means the provision of a range of specialized neonatal pediatric care to address withdrawal symptoms and psychological support for the mother [30].
Breastfeeding provides multiple benefits to the mother and child. In addition to the short-term and long-term medical and neurodevelopmental benefits, it helps in the emotional connection between mother and child, which is particularly important for mothers who are drug users [48]. Breastfeeding should be encouraged in women receiving methadone maintenance treatment; however, for the safety of the infant, the dose should be kept at low levels [49]. The same applies to buprenorphine, which may also be safe during breastfeeding [50]. On the contrary, breastfeeding is contraindicated in cases of cocaine or marijuana use and high doses of benzodiazepines due to potential neurodevelopmental risks [51]. Regarding the infant’s exposure to cannabis contained in the mother’s milk, available recent research data have not shown an increase in neonatal risk; however, infant exposure to cannabis smoke and immediate breastfeeding after cannabis use should be avoided [52].
Conclusions
The therapeutic management of substance use during pregnancy presents a complex clinical and public health challenge. This review highlights that pharmacological substitution therapies, particularly methadone and buprenorphine, can significantly reduce illicit drug use and improve maternal retention in care, although methadone is associated with a higher risk of neonatal abstinence syndrome. Psychosocial interventions, including cognitive behavioral therapy and motivational interviewing, further enhance treatment adherence and reduce relapse rates.
Despite these advances, significant barriers remain, including limited access to integrated services, social stigma, and gaps in healthcare provider training. In Greece, although structured programs exist through organizations such as OKANA and KETHEA, program completion rates remain low, and regional disparities in care persist.
Policy implications include the urgent need for national clinical protocols tailored to pregnant women with substance use disorders, increased funding for regional programs, and standardized interdisciplinary care models. There is also a need to address psychiatric comorbidities systematically, with integrated dual-diagnosis care pathways.
Future research should focus on evaluating the long-term outcomes of various treatment modalities, especially in the Greek context, including maternal-child bonding, developmental outcomes of exposed infants, and health system-level interventions. Developing culturally sensitive, gender-specific interventions and strengthening postnatal care and breastfeeding support are also essential areas for continued study.
Conflicts of Interest
The authors declare that there are no conflicts of interest to report.
Funding
This work was conducted without any specific funding from public, commercial, or nonprofit agencies.
Author Contributions
All authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; drafted the manuscript or revised it critically for important intellectual content; and have approved the final version to be published. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
Not applicable.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the relevant Institutional Review Board / Ethics Committee [name, approval number]. Where applicable, written informed consent was obtained from participants or, in the case of secondary data, the need for consent was waived by the Ethics.
References
- Volkow ND, Blanco C, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2021;22(12):768–80. doi:10.1038/ s41583-021-00512-8
- UNODC (United Nations Office on Drugs and Crime). World Drug Report 2023. Vienna: United Nations Publications; 2023. Available from: https://www.unodc.org/unodc/en/data-and-analysis/wdr2023.html
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2021;72:23–50. doi:10.1146/annurev-psych-010419-050731
- Kring AM, Davison GC, Neale JM, Johnson SL. Psychopathology. Athens: Gutenberg Publications; 2010.
- Vlamaki Z. Interpreting substance dependence [Internet]. PrimeTimes. 2018 Feb; Issue 5 [cited 2025 Jul 8]. Available from: http://www.crimetimes.gr/ermhneyontas-thn-ousioeksarthsh
- World Health Organization (WHO). Dictionary of alcohol and other psychoactive substances. Greek ed. Malliori M-M, editor. Athens: Beta Medical Publications; 2010.
- López-Pelayo H, Miquel L, Teixidor L, Gual A, Balcells MM. The public health impact of legal psychoactive substances: Alcohol, tobacco and caffeine. Int J Environ Res Public Health. 2022;19(15):9214. doi:10.3390/ijerph19159214
- Schifano F, Chiappini S, Corkery JM, Guirguis A. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs. 2020;34(5):491–6. doi:10.1007/s40263-020-00720-4
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). European Drug Report 2022: Trends and Developments. Luxembourg: Publications Office of the European Union; 2022. Available from: https://www.emcdda.europa.eu/publications/edr/trends-developments/2022_en
- Klug H. Legalisation and control: the historical experience of tobacco regulation in England. Soc Hist Med. 2000;13(3):447–63.
- Hall W, Stjepanović D. Legalisation of recreational cannabis: lessons from experience. Lancet Psychiatry. 2023;10(4):263–73. doi:10.1016/S2215-0366(23)00003-1
- World Health Organization (WHO). Global status report on alcohol and health 2021. Geneva: WHO; 2021. Available from: https://www.who.int/publications/i/item/9789240025639
- EMCDDA. Pregnancy, childcare and the family: key issues for Europe’s response to drugs. Selected issue 2012 [Internet]. Lisbon: EMCDDA; 2012 [cited 2025 Jul 8]. Available from: http://www.emcdda.europa.eu/publications/selected-issues/children
- Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167–9.
- EMCDDA. The state of the drug and alcohol problem in Greece: annual report 2018 [Internet]. Athens: URIMH; 2019 [cited 2025 Jul 8]. Available from: https://www.URIMHi.gr/images/Documents/Ethsia-Ekthesh-Ektepn-2018.pdf
- Rolando S, Rena A, O’Neil AL, Beccaria F, Smith CJ. Exploring the level of gender mainstreaming in the working agenda of substance use treatment centers in Italy. Ital J Gender-Specif Med. 2016;2(4):154–7.
- WHO (World Health Organization). Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Geneva: WHO; 2009.
- McIntosh C, Ritson B. Treating depression in substance abuse. Adv Psychiatr Treat. 2001;7 :357–64.
- Liappas I, Paparrigopoulos T, Moussas GI, Mellos E, Karaiskos DA. Therapeutic protocols in the treatment of substance addictions [Internet]. Athens: National Medicines Organization; 2012 [cited 2025 Jul 8]. Available from: http://www.eof.gr/c/document_library/get_file?p_l_id=14016&folderId=236302&name=DLFE-1901.pdf
- Friedell M. Institutional treatment of drug abuse: organization, ideology and outcome. Stockholm: Natur & Kultur; 1996.
- Verheul R. Comorbidity of personality disorders in individuals with substance use disorders. Eur Psychiatry. 2001;16:274–82.
- Lauritzen I, De Weille JR, Lazdunski M. The potassium channel opener (-)-cromakalim prevents glutamate-induced cell death in hippocampal neurons. J Neurochem. 1997;69(4): 1570–9.
- Beutel M. Addiction and sexual abuse. Psychotherapist. 1999;44:313–9.
- Kelly TM, Daley DC. Integrated treatment of substance use and psychiatric disorders. Soc Work Public Health. 2020;35(3):85–98. doi:10.1080/19371918.2020.1734040
- McGovern MP, Lambert-Harris C, Gotham HJ, Claus RE, Xie H. Dual diagnosis capability in mental health and addiction treatment services: A review and roadmap. Psychiatr Serv. 2021;72(6):636–44.doi:10.1176/appi.ps.2020 00450
- Marel C, Mills KL, Kingston R, Kay-Lambkin F, Teesson M. Comorbidity of mental and substance use disorders: a systematic review of longitudinal studies. Lancet Psychiatry. 2023;10(1):40–55. doi:10.1016/S2215-0366 (22)00310-6
- Papadimitriou GN, Economou M, Palli A, Tsapakis EM. Challenges in the treatment of dual diagnosis patients in Greece: Service fragmentation and stigma. Int J Ment Health Syst. 2022;16(1):42. doi:10.1186/s13033-022-00544-2
- Priester MA, Browne T, Iachini A, Clone S, DeHart D, Seay KD. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: An integrative literature review. J Subst Abuse Treat. 2016;61:47–59. doi:10.1016/j.jsat. 2015.09.006
- Goettler SM, Tschudin S. Care of drug-addicted pregnant women: current concepts and future strategies—an overview. Womens Health (Lond). 2014;10(2):167–77.
- OKANA (Organization Against Drugs). Programs for pregnant women who use substances. Athens: OKANA; 2013.
- KETHEA. United Nations International Narcotics Control Board: annual report 2019 [Internet]. Athens: KETHEA; 2019 [cited 2025 Jul 8]. Available from: https://www.kethea.gr/wp-content/uploads/2020/02/KETHEA-OHE-2019_Lowres.pdf
- Stone R, Bauer AM, Carson A. Improving maternal outcomes in pregnant women with substance use disorders. Obstet Gynecol Clin North Am. 2022;49(1):31–44. doi:10.1016/j.ogc. 2021.11.005
- Jones HE, Short E. Pregnancy and substance use: treatment strategies and outcomes. Curr Opin Psychiatry. 2021;34(4):348–53. doi:10.1097/ YCO.0000000000000697
- ACOG (American College of Obstetricians and Gynecologists). Opioid Use and Opioid Use Disorder in Pregnancy. Committee Opinion No. 711. Obstet Gynecol. 2020;130(2):e81–94. doi:10.1097/AOG.0000000000003055
- Black M, Day E. Comprehensive care for pregnant women with substance use disorders: best practices and challenges. J Subst Abuse Treat. 2023;145: 108901. doi:10.1016/j.jsat.2022.108901
- Moses J, Roach MJ, Cheetham M. Providing equitable care to pregnant women who use substances: challenges and opportunities. Midwifery. 2021;98:102951. doi:10.1016/j. midw.2021.102951
- DHDA (Drug Harm and Dependency Agency). Guidelines for the treatment of pregnant women with substance use disorders. Athens: DHDA; 2007.
- Huybrechts KF, Kogut K, Bartels DB, Lund JL, Bateman BT. Trends in the use of prescription opioids during pregnancy in the United States, 2000–2015. Pharmacoepidemiol Drug Saf. 2020;29(3):298–305. doi:10.1002/pds.4915
- Marsden J, Stillwell G, Jones H, Cooper A. Integrated care for pregnant women with substance use disorders: a review. Addiction. 2021;116(6):1380–92.doi:10.1111/add.15 255
- New Zealand Ministry of Health (NZMH). Guidelines for the management of substance use in pregnancy. Wellington: NZMH; 2008.
- Lundgren LM, Amaro H, Hussain F, Vega M. Integrated treatment approaches for pregnant and parenting women with substance use disorders: A review. J Subst Abuse Treat. 2021; 129:108374. doi:10.1016/j.jsat.2021.108374
- Smith L, Richardson L, Hoffman K, Reese B. Multidisciplinary care models and outcomes for pregnant women with opioid use disorder. Substance Abuse. 2020;41(4):501–8. doi:10.1080/ 08897077.2020.1784453
- National Institute for Health and Clinical Excellence (NICE). Pregnancy and complex social factors: a model for service provision for pregnant women with complex social factors. London: NICE; 2010.
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). Treatment of opioid dependence with methadone and buprenorphine. Lisbon: EMCDDA; 2012. Available from: https://www.emcdda.europa.eu/publications
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Buprenorphine treatment of pregnant opioid-dependent women: a comprehensive review. Addiction. 2019;114(2):235 -46. doi:10.1111/add.14441
- Scully IL, Walter MA, Nolan M, Burns L, O’Leary CM, Bower C. Methadone dosage and neonatal abstinence syndrome – a population study. Drug Alcohol Depend. 2004;76(3):277–82. doi:10.1016/j.drugalcdep.2004.05.001
- Winklbaur B, Baewert A, Jung EM, Fischer G, Unterrainer HF. Pharmacological treatment of opioid-dependent pregnant women and neonatal abstinence syndrome. Curr Opin Psychiatry. 2021;34(4):347–51. doi:10.1097/YCO.00000000 00000696
- Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. doi:10.1016/S0140-6736(15)01024-7
- Kronfli N, et al. Safety of Methadone Maintenance Treatment in Breastfeeding Women: A Systematic Review. Addiction. 2021;116(10) :2714–26. doi:10.1111/add.15498
- Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB, Benjamin DK. Increasing Incidence of the Neonatal Abstinence Syndrome in U.S. Neonatal Intensive Care Units. Pediatrics. 2019;143(3):e20181018. doi:10.1542/peds. 2018-1018
- Holmes A, Atkins R, Fisher P. Benzodiazepine use and breastfeeding outcomes: a systematic review. Eur J Pediatr. 2022;181:87–97. doi:10.1007 /s00431-021-04167-7
- Cooper ZD, Haney M, Magee LA. Cannabis Use and Breastfeeding: What We Know and What We Need to Know. Pediatrics. 2022;149(2): e2021054321. doi:10.1542/peds.2021-054321