Research
HJOG 2025, 24 (4), 222-232| doi: 10.33574/hjog.0603
Eleni Louri, Antigoni Tranidou, Apostolos Mamopoulos, Themistoklis Dagklis, Ioannis Tsakiridis
Third Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Correspondence: Ioannis Tsakiridis, Konstantinoupoleos 49, 54642, Thessaloniki Tel: +30 2313312120 and Fax: +30 2310 992950, e-mail: iotsakir@gmail.com
Abstract
Introduction: Gestational diabetes mellitus (GDM) is a metabolic complication of pregnancy first diagnosed during pregnancy. It can cause complications like preterm birth, cesarean delivery, macrosomia and postpartum type 2 diabetes mellitus. Lifestyle changes, obesity and older age of pregnant women are considered risk factors for developing GDM.
Material and Methods: This was a retrospective study conducted at 3rd Department of Obstetrics and Gynecology, Aristotle University of Thessaloniki, Greece, between January 2023 and December 2024. A linear regression model, adjusted for maternal characteristics, was used to analyze BMI and weight gain differences between women diagnosed with GDM and those without GDM. Specifically, the study examined these differences between 28 to 35 weeks of gestation after GDM diagnosis. Additionally, the study assessed the impact of BMI and weight gain up to 28 weeks of pregnancy on the risk of developing GDM, using a logistic regression model.
Results: A total of 149 women were eligible for the study. Among them 32.2% were diagnosed with GDM. Women that developed GDM exhibited higher increase in BMI (median change: 1.98 [1.56, 3.04] vs. 1.1 [0.77, 1.45], p < 0.0001), and also showed higher weight gain (median weight gain: 5.5 [4, 8] kg vs. 3 [2, 4] kg, p < 0.0001) between 28 and 35 weeks of pregnancy. Women with GDM had significantly higher pre-pregnancy weight, weight at 28 and 35 weeks, and BMI before pregnancy, compared to non-GDM women (all p < 0.0001). A significantly higher proportion of women with GDM conceived via IVF (16.67% vs. 3.96%, p = 0.019). Women with higher BMI at 28 weeks of pregnancy had 51% lower odds of developing GDM (aOR = 0.49, 95% CI: 0.28–0.82, p = 0.009). Women who gained weight below IOM guidelines had 158% higher odds of developing GDM (aOR = 2.58, 95% CI: 1.13–6.04, p = 0.026), while those who gained weight within IOM guidelines had 63% lower odds. (aOR = 0.37, 95% CI: 0.15–0.86, p = 0.025). Additionally, from 28 to 35 weeks after GDM diagnosis, women with GDM gained an average of 3.34 kg more weight (p = 3.11 × 10⁻¹⁴) and had a 1.2 units higher BMI (p = 3.46 × 10⁻¹⁴) than non-GDM women.
Conclusion: Pre-pregnancy healthy body weight and weight gain within the recommendation limits is the key to reduce the risk of GDM. Close monitoring and targeted interventions to monitor BMI and weight changes after GDM diagnosis, is crucial to improve pregnancy outcomes.
Keywords: Gestational diabetes mellitus, gestational weight gain, BMI in pregnancy, BMI change, weight change.
Introduction
Gestational diabetes mellitus (GDM) is a common metabolic complication of pregnancy characterized by hyperglycemia, which results from increased insulin resistance and reduced insulin secretion [1]. It is usually diagnosed during the late second trimester of pregnancy in women who did not have diabetes prior to pregnancy. It increases the risk of adverse pregnancy outcomes, such as preterm birth, cesarean delivery, macrosomia, and postpartum type 2 diabetes mellitus and cardiovascular disease in the future [2, 3]. In addition, infants born to mothers with GDM are more like to be obese and develop type two diabetes later in life [4]. GDM is reported to complicate up to 25% of pregnancies globally due to lifestyle changes, growing incidence of obesity, and older age of pregnant women [5, 6].
Previous studies have mentioned potential risk factors for GDM [7]. These include genetic and environmental factors [8], advancing maternal age, increasing pre-pregnancy body mass index (BMI), increasing parity, having a previous macrosomic neonate, family history of diabetes, polycystic ovarian syndrome (PCOS), and smoking [9, 10]. Excessive gestational weight gain (GWG) is highly prevalent in women with GDM [11]. It has been established that inappropriate GWG is an independent risk factor of pregnancy-related morbidity. Excessive GWG is associated with perinatal and maternal complications, including gestational diabetes, fetal macrosomia, preeclampsia, instrumental delivery and peripartum infections [12, 13]. Many studies support that overweight and obesity before pregnancy and an excessive GWG are associated with a greater risk of developing GDM [14-16]. Compared to body weight alone, BMI has the advantage that it also considers height, providing a more comprehensive assessment of a person’s body composition throughout the whole pregnancy. Pre-pregnancy BMI has been implicated as the main predictor of GDM [17].
According to IOM guidelines [18], recommended BMI and weight gain between 28 and 35 weeks of pregnancy vary based on pre-pregnancy weight categories. While higher pre-pregnancy BMI is a well-established risk factor for GDM, there is limited research on how BMI and weight gain progress after GDM diagnosis (28–35 weeks) compared to non-GDM women. Additionally, the association between BMI changes and weight gain up to 28 weeks and the risk of developing GDM remains unclear.
The aim of this study was to compare the BMI and weight changes between women diagnosed with GDM and those without GDM at 28-35 weeks of pregnancy. In addition, the correlation between BMI changes until 28 weeks of gestation and onset of GDM, was also investigated.
Material and methods
Study design and population
This retrospective case-control study was conducted at the 3rd Department of Obstetrics and Gynecology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Greece, between January 2023 and December 2024. Written informed consent was obtained from all participants for the use of their anonymized data for research purposes, with no incentives offered. In accordance with standard protocols for observational studies that do not involve interventions or modifications to routine patient care (https://www.hra.nhs.uk/approvals-amendments/what-approvals-do-i-need/), the study was exempt from institutional board review [19].
Inclusion and exclusion criteria
Eligible participants were those with complete data regarding their weight measurements at the followed time points: pre-pregnancy, 28 weeks (diagnosis’ point) and 35 weeks of pregnancy. Exclusion criteria included: i) maternal age <18 years, ii) presence of serious pre-existing medical conditions, including chronic hypertension or pre-existing diabetes, iii) multiple gestations (e.g., twins or higher-order multiples), iv) insufficient or missing data on key weight measurements or GDM diagnosis, v) women who did not consent to the use of their data for research purposes.
Data collection and variables
Data were collected from the participants’ medical records and included information on their obstetric and medical history, age, height, weight, and gestational age. To diagnose GDM, all women underwent a 75 g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation, according to the guidelines provided by the Hellenic Society of Obstetricians and Gynecologists (HSOG) [20], which are based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [21]. GDM was diagnosed if at least one of the blood glucose measurements met or exceeded the following thresholds: (i) fasting ≥92 mg/dL, (ii) 1-hour ≥180 mg/dL, (iii) 2-hour ≥153 mg/dL.
BMI was calculated for each participant at three time points: pre-pregnancy, 28 weeks of pregnancy, and 35 weeks of gestation. The primary objective was to compare the BMI changes between women diagnosed with GDM and those without GDM at 28 to 35 weeks of pregnancy. Secondary analyses explored the relationships between GDM and various pregnancy-related factors, including BMI.
To classify weight gain during pregnancy, the Institute of Medicine (IoM) guidelines were used, which provide recommended weight gain ranges based on pre-pregnancy BMI. According to these guidelines, the recommended weight gain ranges up to the 28th gestational week are as follows: for underweight individuals (BMI < 18.5), 7–10 kg; for normal-weight individuals (BMI 18.5–24.9), 5.5–8 kg; for overweight individuals (BMI 25–29.9), 3.5–6 kg; and for obese individuals (BMI ≥ 30), 2.5–5 kg. Using these thresholds, weight gain was classified into three categories: below, within, or above the IoM recommendations. BMI was calculated using the height (m) and weight (kg) of participants in the formula BMI = kg/m2. Moreover, for weight gain at 28 to 35 weeks of pregnancy the recommended weight gain ranges from 28 to 35 weeks are as follows: for underweight individuals (BMI < 18.5), 3–4 kg; for normal-weight individuals (BMI 18.5–24.9), 2.5–3.5 kg; for overweight individuals (BMI 25–29.9), 1.5–2.5 kg; and for obese individuals (BMI ≥ 30), 1–2 kg.
In addition, plots were created to visually explore the relationships between previous GDM history, current GDM status, and mean BMI at different stages of pregnancy. The objective was to identify trends and potential differences in BMI across various GDM groups, including non-GDM women, women with a history of GDM, and women with current GDM.
All relevant data were securely stored in a local database maintained at the 3rd Department of Obstetrics and Gynecology, Aristotle University of Thessaloniki, and maintained in compliance with data protection and privacy regulations.
To assess the normality of continuous variables, the Shapiro–Wilk test was used. For normally distributed variables the data were presented as means with standard deviations (SDs), while non-normally distributed variables were summarized as medians with interquartile ranges (IQRs). Categorical variables were reported as frequencies and percentages. Group comparisons were made using the Chi-square or Fisher’s exact test, as appropriate. Statistical analysis was carried out using R (version 4.2.1). Based on the normality of the data, comparisons between groups were performed using t-tests for normally distributed variables, and the Mann–Whitney U test for non-normally distributed variables.
To assess the association between weight gain, BMI and GDM, a logistic regression model was applied to calculate adjusted odds ratios (aORs) with 95% confidence intervals (CIs). Covariates included maternal age, BMI before pregnancy, previous GDM history, conception method (IVF or spontaneous), parity, smoking status.
To calculate BMI differences between GDM and non-GDM individuals after GDM diagnosis, at 28 to 35 weeks of gestation a linear regression model was used. The weight gain differences were also computed. The model was adjusted for history of GDM, conception method, maternal age, parity, and smoking.
Results
Data from 149 women were retrospectively collected, with 32.2% of them developing GDM (101 non-GDM women, 48 women with GDM). Maternal characteristics of the two groups are shown in Table 1. The mean pre-pregnancy weight was significantly higher in the GDM group compared to the non-GDM group (79.5 [66, 89.5] vs. 67 [60, 72] kg, p < 0.0001). Similarly, the mean weight at 28 weeks (80.5 [70.75, 92.75] vs. 72 [65, 78] kg, p < 0.001) and at 35 weeks (88 [77, 98] vs. 75 [69, 81] kg, p < 0.0001) were both significantly higher in women with GDM. The mean pre-pregnancy BMI was also higher in the GDM group (28.71 [24.45, 34.51] vs. 24.45 [22.04, 26.33], p < 0.0001), and this trend continued at 28 weeks (29.56 [26.46, 35.15] vs. 26.45 [24.16, 28.58], p < 0.001) and at 35 weeks of pregnancy (31.55 [28.2, 37.05] vs. 27.61 [25.34, 29.75], p < 0.0001).
Additionally, women with GDM experienced significantly greater changes in both BMI and weight between 28 and 35 weeks of pregnancy. Specifically, the median change in BMI in the GDM group was 1.98 [1.56, 3.04], compared to 1.1 [0.77, 1.45] in non-GDM women. Similarly, the median change in weight gain was 5.5 [4, 8] kg in the GDM group versus 3 [2, 4] kg in the non-GDM group (p < 0.0001 for both comparisons). No significant differences were observed between the groups for maternal age (34 [29, 38] vs. 35 [31, 36] years, p = 0.85), height (164.69 ± 5.32 vs. 165.17 ± 4.23 cm, p = 0.55), smoking (8.33% vs. 3.96%, p = 0.47), or previous GDM history (6.25% vs. 2.97%, p = 0.61).
The proportion of women conceiving via in vitro fertilization (IVF) was significantly higher in the GDM group (16.67% vs. 3.96%, p = 0.019), while the proportion of women who conceived naturally was higher in the non-GDM group (96.04% vs. 83.33%, p = 0.019). There were no significant differences in parity between the two groups.
Table 2 reports the association between weight gain and BMI status at 28 weeks of pregnancy and the risk of GDM. Women who gained weight below the IOM guidelines had significantly higher odds of developing GDM (aOR = 2.58, 95% CI: 1.13, 6.04, p = 0.026). Conversely, women who gained weight within the IOM guidelines had significantly lower odds of developing GDM (aOR = 0.37, 95% CI: 0.15, 0.86, p = 0.025). Notably, no significant association was found for women who gained above the IOM guidelines (aOR = 1.02, 95% CI: 0.28, 3.29, p = 0.97).
At 28 weeks of pregnancy, women with a higher BMI had significantly lower odds of developing GDM (aOR = 0.49, 95% CI: 0.28, 0.82, p = 0.009).
Table 3 presents differences in BMI and weight gain pattern during the 28 and 35 weeks of pregnancy, in women diagnosed with GDM, in comparison to women without GDM. Regarding BMI differences, women diagnosed with GDM, on average has a 1.2 higher total BMI during this period compared to women without a GDM diagnosis (p=3.46 × 10⁻¹⁴). Regarding weight gain differences, women diagnosed with GDM gain on average an additional 3.34 kg in total during 28-35 weeks of pregnancy, compared to women without GDM (p=3.11 × 10⁻¹⁴).
Figure 1 demonstrates the relationship between previous GDM history and current GDM status with mean pre-pregnancy BMI. Non-GDM women without a previous history of GDM have a lower pre-pregnancy BMI (around 24 to 26) compared to non-GDM women with history of GDM. Women with current GDM and a previous history of GDM exhibit no difference in BMI before pregnancy compared to GDM women without a history of previous GDM. The difference in BMI between the non-GDM women with no previous history of GDM and current GDM with no previous GDM history is more pronounced compared to the non-GDM women with a history of GDM and current GDM women with a history of GDM.
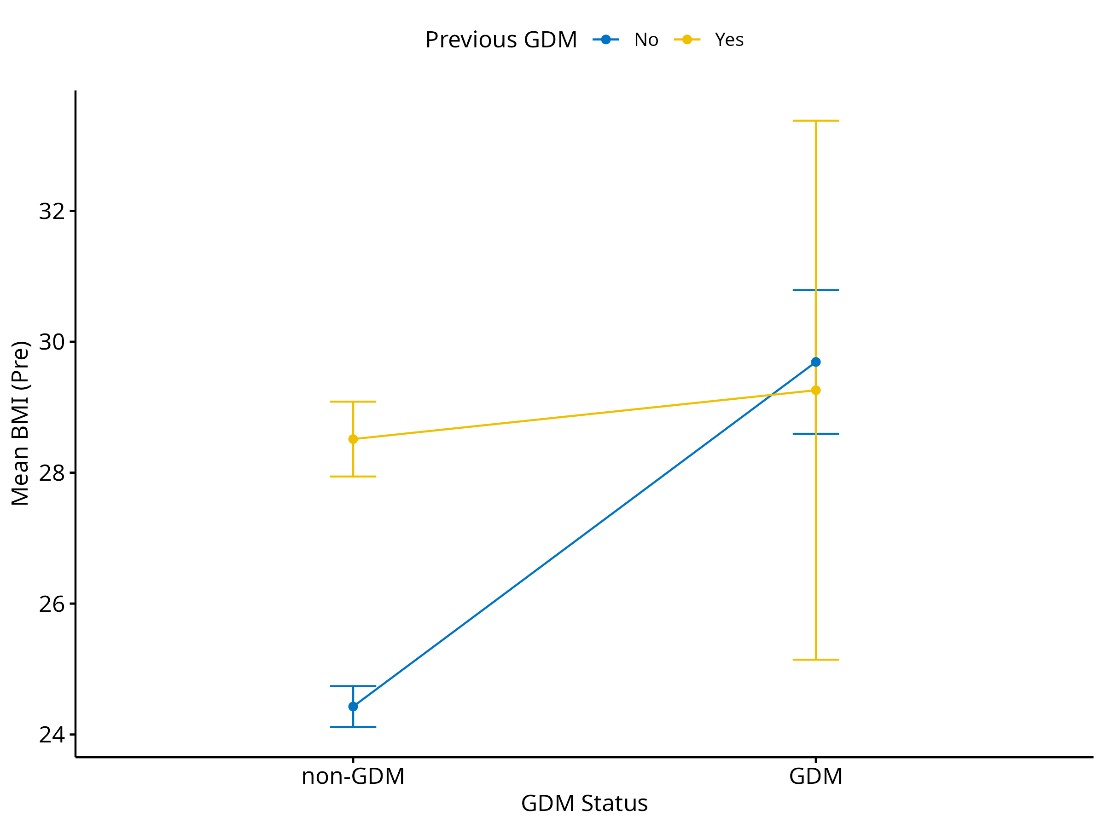
Figure 1. Mean BMI before pregnancy by GDM status and previous GDM history.
BMI (Pre): body mass index before pregnancy; GDM: gestational diabetes mellitus.
Figure 2 illustrates the relationship between previous GDM history, current GDM status, and mean BMI at 28 weeks of pregnancy. Non-GDM women without a history of GDM tend to have lower BMI (around 26 to 27) compared to non-GDM women with a history of GDM. Women with current GDM and a previous history of GDM have no difference in BMI at 28 weeks of pregnancy compared to GDM women without a history of previous GDM. The difference in BMI between the non-GDM women with no previous history of GDM and current GDM with no previous GDM is more pronounced compared to the non-GDM women with a history of GDM and current GDM women with a history of GDM.
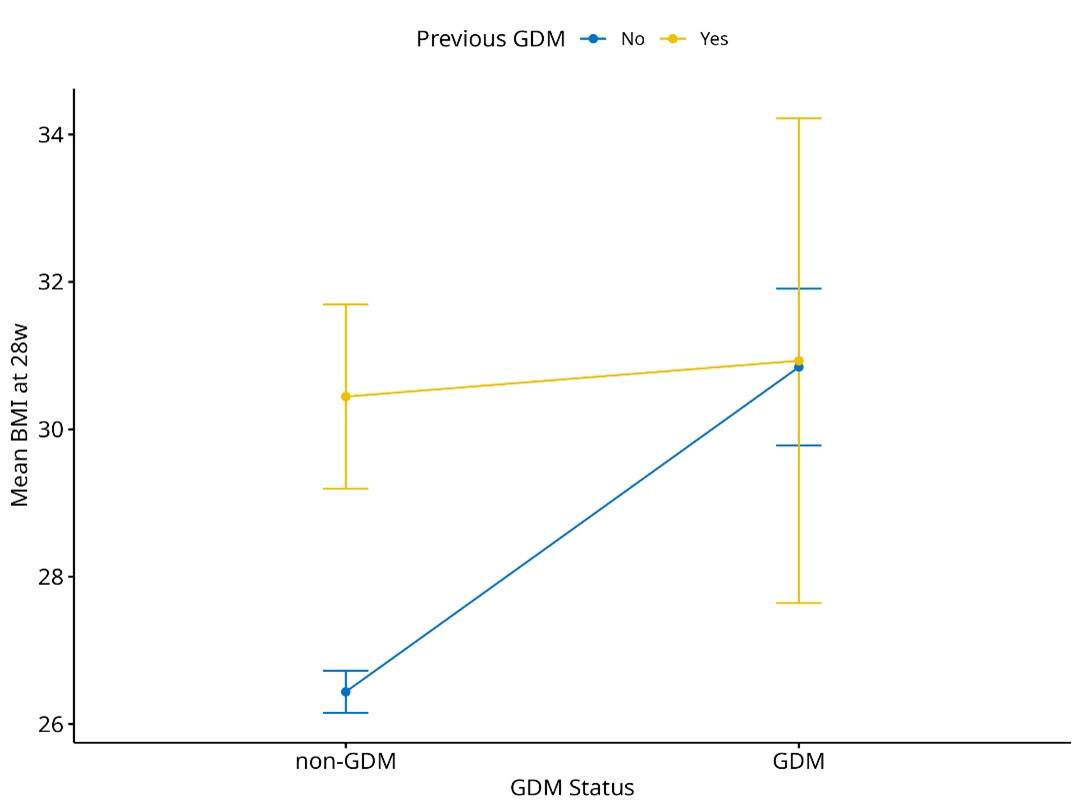
Figure 2. Mean BMI at 28 weeks of pregnancy by GDM status and previous GDM history.
BMI: body mass index; GDM: gestational diabetes mellitus.
Figure 3 shows the relationship between previous GDM history, current GDM status, and mean BMI at 35 weeks of pregnancy. Non-GDM women without a previous history of GDM have lower BMI at 35 weeks of pregnancy (around 27.5 to 28) compared to non-GDM women with history of GDM. Women with current GDM and a previous history of GDM show no difference in BMI at 35 weeks of pregnancy compared to GDM women without a history of previous GDM. The difference in BMI between the non-GDM women with no previous history of GDM and current GDM with no previous GDM history is more pronounced compared to the non-GDM women with a history of GDM and current GDM women with a history of GDM.
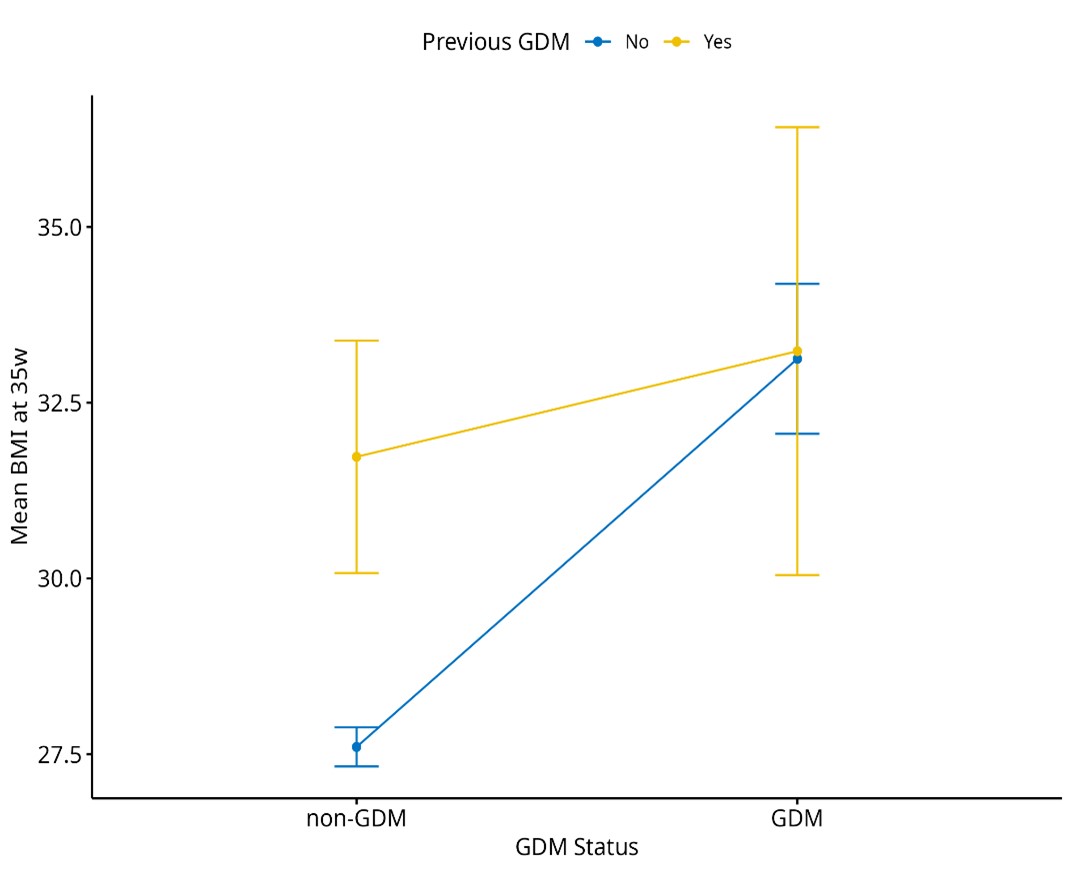
Figure 3. Mean BMI at 28 weeks of pregnancy by GDM status and previous GDM history.
BMI: body mass index; GDM: gestational diabetes mellitus.
Figure 4 illustrates the mean change in BMI from pre-pregnancy to 28 weeks and 35 weeks of pregnancy for non-GDM and GDM women. Non- GDM women at 28 weeks of pregnancy seem to have higher BMI change (around 2 units) compared to GDM women (around 1.2 units). At 35 weeks of pregnancy this reverses, women with current GDM have higher BMI increase compared to women without GDM.
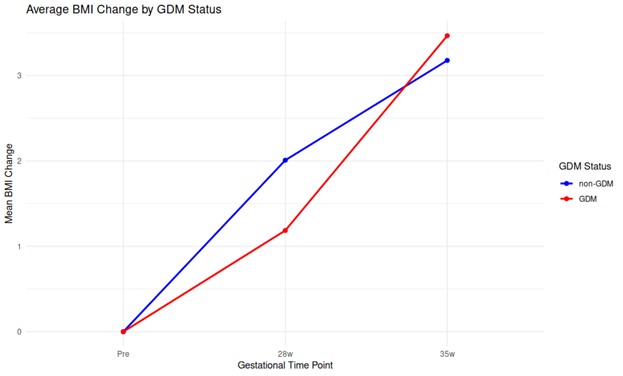
Figure 4. Average BMI changes by GDM status.
BMI: body mass index; GDM: gestational diabetes mellitus.
Discussion
Main findings
The study’s findings are as follows: 1) women with GDM experienced increase in BMI at 28 to 35 weeks of pregnancy, 2) women with GDM demonstrated higher weight gain at 28 to 35 weeks of pregnancy, 3) at 28 to 35 weeks of pregnancy women diagnosed with GDM had on average 1.2 units higher BMI compared to women without GDM, 2) at 28 to 35 weeks of pregnancy women diagnosed with GDM gained on average 3.34 kg more weight than their non-GDM counterparts, 4) higher pre-pregnancy weight and BMI were more common in women with GDM, 5) women that developed GDM had higher weight and BMI at 28 and 35 weeks of pregnancy, compared to women that did not develop GDM, 6) women with conception via IVF were more likely to develop GDM and 7) women who gained weight within IOM guidelines until 28 weeks of pregnancy had lower odds of developing GDM.
Regarding the plot analysis, 8) non-GDM women without a history of GDM generally have lower BMI compared to those with a history of GDM, and 9) there is no significant difference in BMI between women with current GDM and those with both a previous and current GDM diagnosis at pre-pregnancy, 28 weeks, and 35 weeks of pregnancy. Additionally, 10) non-GDM women seem to experience a higher BMI increase from pre-pregnancy to 28 weeks, while women with current GDM show a higher increase from 28 weeks to 35 weeks. These findings suggest trends in BMI differences between the groups at various stages of pregnancy. However, due to the nature of the analysis based on plots, caution should be exercised in drawing firm conclusions, and further statistical analysis may be necessary to confirm the significance of these trends.
Interpretation of the findings
A main finding of the current study was that women with a diagnosis of GDM experienced higher BMI and weight gain increase between 28 and 35 weeks of pregnancy, compared to women without a GDM diagnosis. A study by Cwiek et al. [22] found no significant differences in total gestational weight gain (GWG) between women with and without GDM. However, they reported that among overweight and obese women, those with GDM had a higher percentage of weight gain during pregnancy. Similarly, Yang et al. [23], observed that higher pre-pregnancy BMI was associated with excessive GWG in GDM mothers, but neither study specifically analyzed weight gain after GDM diagnosis. Conversely, Morisset et al. [24], reported that women with GDM gained significantly more weight in the first trimester than non-GDM women, but experienced lower weight gain in the third trimester. This contrasts with our findings, where non GDM women showed a greater BMI increase from pre-pregnancy to 28 weeks, whereas women with GDM exhibited a greater BMI and weight gain increase from 28 to 35 weeks. These differences suggest that weight gain patterns may shift post-GDM diagnosis, potentially due to metabolic changes or clinical interventions. Further supporting the variability in weight gain patterns, Miao et al. [25] found that women with GDM had lower total pregnancy weight gain compared to those with normal glucose tolerance and exhibited a reduced weight gain rate after the OGTT. Their findings indicate that GDM women had a higher likelihood of insufficient weight gain (41.1% vs. 10.4%) and a lower likelihood of excessive weight gain (22.6% vs. 54.2%). While these results suggest that GDM may slow down weight progression after diagnosis, our study specifically examined the 28–35 week’ period, where we observed that GDM women had significantly greater BMI and weight gain increases than non-GDM women. This discrepancy highlights the importance of evaluating weight gain trajectories at different gestational periods rather than considering pregnancy as a single phase.
Appropriate weight gain during pregnancy has been investigated since the early 1930s [26]. The ideal weight gain in pregnancy has changed in time. The most recent recommendations of The Institute of Medicine (IOM) in 2009 introduced the ideal weight gain in pregnancy according to the pre-pregnancy BMI of pregnant women [18].
The present study noted that women with GDM had higher pre-pregnancy weight and BMI compared to non-GDM women. This finding is consistent with findings from a systematic review and meta-analysis, which found that the risk of GDM was significantly greater in overweight and obese women (23%) compared to those with normal or underweight BMI (10.7%) [27]. Moreover, our study found that women that gain weight within the IOM guidelines around the screening at 28 weeks have reduced risk of developing GDM .This contraindicated to a study suggesting that there were no differences in gestational gain weight before GDM screening, between women with and without GDM so that no significant association was found between excessive GWG (weight gain above the 90th percentile of the study population or exceeding the upper range recommended by IOM) up to GDM screening, and later development of GDM [28]. Interestingly, our study found that women with higher BMI up to 28 weeks of pregnancy were associated with a reduced risk of GDM, this contraindicates a study which reports early gestational weight gain below average was associated with a decreased risk for GDM in obese women [29]. This reverse finding may be attributed to the fact that a subset of women was recruited from a high-risk clinic, affecting BMI distribution. A key observation in this study was that a large number of GDM cases were classified as overweight (41%) and obese (3.96%), which may have significant implications for the relationship between BMI changes and GDM risk. Obesity is a well-established risk factor for GDM, as excess adiposity contributes to increased insulin resistance and metabolic dysregulation [30]. Pre-pregnancy obesity often leads to dietary and lifestyle modifications, which can affect weight gain patterns, potentially confounding the observed associations. However, more investigation is needed with further studies to clarify this relationship.
Studies have prioritized the achievement of the ideal BMI before conception and preventing excessive gain weight [31, 32]. Pre-gestational obesity is the most challenging part as lifestyle changes have to occur before conception. Maintaining a recommended weight gain seems to be more easily implemented by women.
Additionally, in this study’s population, a higher percentage of women with GDM conceived via IVF. This is consistent with previous studies [33], and even though after adjusting for maternal age, the association persisted, suggesting that factors beyond age may contribute to the increased risk for GDM among women conceiving via IVF.
According to the findings from the plot analysis in the current study women with GDM tend to experience a lower BMI increase and more rapidly during 28 and 35 weeks of gestation in comparison with women that did not develop GDM, who experienced higher BMI increase up to the GDM screening around 28 weeks, and then the increase was lower. Some researchers have declared that restricting GWG in women with GDM may benefit pregnancy outcomes [34], emphasizing the importance of encouraging a reduced GWG, provided adequate fetal growth is maintained, and suggesting that IOM recommendations should be reevaluated specifically for different BMI populations.
However, since these findings are derived visually from plot analyses, definitive conclusions cannot be drawn, and further studies are needed. Additionally non-GDM-women without a history of GDM generally tent to have lower BMI compared to those with a history of GDM [35]. In addition to the above, the plot analysis revealed there is no significant difference in BMI between women with current GDM and those with both a previous and current GDM diagnosis at pre-pregnancy, 28 weeks, and 35 weeks of pregnancy. Studies do not clearly differentiate this effect between these groups, so more research is needed to clarify these BMI trajectories.
This study benefits from a centralized recruitment (single department) ensured consistency in data collection and the application of standardized diagnostic criteria for GDM. Moreover, the adjusting for the control key confounders, such as pre-pregnancy BMI, maternal age, and previous history of GDM, enhanced the reliability of the findings. One of the study’s limitations is the retrospective design and the relatively small sample size, which may limit the generalizability of the results beyond the Greek population, as well as the lack of information on lifestyle factors like diet and physical activity that are known to affect GDM risk. Furthermore, the inclusion of GDM cases from a high-risk clinic may have introduced selection bias, potentially impacting the results.
Conclusion
This report has shown the relevance of BMI changes in the first 28 weeks of pregnancy when GDM is usually diagnosed. Pre-pregnancy high BMI was associated with the risk of developing GDM. Women with GDM gained more weight post diagnosis, compared to non-GDM women. Women with previous GDM tent to have higher BMI during subsequent pregnancies. The key of not developing GDM is a healthy weigh before pregnancy.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
Νo funding was received for this study.
References
- Association AD. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35:S64.
- Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377.
- Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Inter Med. 2017;177:1735-42.
- Lowe Jr WL, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42:372-80.
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141.
- Melchior H, Kurch-Bek D, Mund M. The prevalence of gestational diabetes: a population-based analysis of a nationwide screening program. Dtsch Arztebl Int. 2017;114:412.
- Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. 2016;59:1385-90.
- Popova PV, Klyushina AA, Vasilyeva LB, et al. Association of common genetic risk variants with gestational diabetes mellitus and their role in GDM prediction. Front Endocrinol (Lausanne). 2021;12:628582.
- Leng J, Shao P, Zhang C, et al. Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: a prospective population-based study in Tianjin, China. PloS one. 2015;10:e0121029.
- Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:1-20.
- Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstetrics & Gynecology. 2014;123:737-44.
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. International Journal of Gynecology & Obstetrics. 2006;93:269-74.
- Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115: 597-604.
- Brunner S, Stecher L, Ziebarth S, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia. 2015;58:2229-37.
- Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118: 305-12.
- Leng J, Li W, Zhang S, et al. GDM women’s pre-pregnancy overweight/obesity and gestational weight gain on offspring overweight status. PloS one. 2015;10:e0129536.
- Torloni M, Betrán A, Horta B, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta‐analysis. Obes Rev. 2009;10:194-203.
- Yaktine AL, Rasmussen KM. Weight gain during pregnancy: reexamining the guidelines. 2010.
- Wade DT. Ethics, audit, and research: all shades of grey. BMJ. 2005;330:468-71.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Diagnosis and Management of Gestational Diabetes Mellitus: An Overview of National and International Guidelines. Obstet Gynecol Surv. 2021;76:367-81.
- Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202:654. e1-. e6.
- Ćwiek D, Lubkowska A, Zimny M, Szymoniak K, Sipak-Szmigiel O. Weight Gain during and after Pregnancy in Women with Gestational Diabetes Mellitus-A Preliminary Study. International Journal of Environmental Research and Public Health. 2022;19:11959.
- Yang Y, Wei Q, Yu H, et al. Higher pre‐pregnancy body mass index is associated with excessive gestational weight gain in normal weight Chinese mothers with gestational diabetes. J Obstet Gynaecol Res. 2016;42:511-8.
- Morisset A-S, Tchernof A, Dubé M-C, Veillette J, Weisnagel SJ, Robitaille J. Weight gain measures in women with gestational diabetes mellitus. J Womens Health (Larchmt). 2011;20:375-80.
- Miao H, Liang F, Zheng Z, et al. Weight progression and adherence to weight gain target in women with vs. without gestational diabetes: a retrospective cohort study. BMC Pregnancy Childbirth. 2023;23:513.
- Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutr. 2008;28:411-23.
- Najafi F, Hasani J, Izadi N, et al. Risk of gestational diabetes mellitus by pre-pregnancy body mass index: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021;15:102181.
- Chuang YC, Huang L, Lee WY, Shaw SW, Chu FL, Hung TH. The association between weight gain at different stages of pregnancy and risk of gestational diabetes mellitus. Journal of Diabetes Investigation. 2022;13:359-66.
- Xu H, Hutcheon JA, Liu X, et al. Risk of gestational diabetes mellitus in relation to early pregnancy and gestational weight gain before diagnosis: A population‐based cohort study. Acta Obstet Gynecol Scand. 2022;101:1253-61.
- Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315.
- Zehravi M, Maqbool M, Ara I. Correlation between obesity, gestational diabetes mellitus, and pregnancy outcomes: an overview. Int J Adolesc Med Health. 2021;33:339-45.
- Tranidou A, Magriplis E, Tsakiridis I, et al. Effect of Gestational Weight Gain during the First Half of Pregnancy on the Incidence of GDM, Results from a Pregnant Cohort in Northern Greece. Nutrients. 2023;15.
- Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. 2014;176:149-52.
- Ferreira JL, Voss G, Dória M, Couto AS, Príncipe RM. Benefit of insufficient gestational weight gain in obese women with gestational diabetes mellitus: A multicenter study in Portugal. Diabetes Metab Syndr: Clinical Research & Reviews. 2021;15:419-24.
- Fan Y, Li W, Liu H, et al. Effects of obesity and a history of gestational diabetes on the risk of postpartum diabetes and hyperglycemia in Chinese women: Obesity, GDM and diabetes risk. Diabetes Res Clin Pract. 2019;156:107828.