Research
HJOG 2025, 24 (3), 160-169 | doi: 10.33574/hjog.0602
Rukhsar Majeed1, Syed Amir Gilani3, Syed Muhammad Yousaf Farooq2, Meryam Zulfiqar4
1University Institute of Radiological Sciences and Medical Imaging Technology, The University of Lahore, Pakistan.
2Department of Medical Diagnostic Imaging, College of Health Sciences, University of Sharjah, United Arab Emirates.
3Department of Radiography and Imaging Technology, Green International University, Lahore.
4Obstetrics and gynecological Department, Sulaiman Al Habib Sahafa, Saudia Arabia.
Correspondence: Syed Muhammad Yousaf Farooq, MPhil., PhD. Department of Radiography and Imaging Technology, Green International University, Lahore. Email: Yousafgelani@gmail.com, ORCID: https://orcid.org/0000-0001-5768-224X
Abstract
Objective: This study aimed to examine the hemodynamic changes in the umbilical artery among third-trimester pregnant women with oligohydramnios, gestational hypertension, and both conditions.
Materials and Methods: A case-control study was conducted over nine months, enrolling 88 women in their third trimester. Participants were categorized into four groups: (1) normal, (2) oligohydramnios, (3) gestational hypertension, and (4) oligohydramnios with gestational hypertension. Doppler ultrasound was used to assess umbilical artery resistive index (UA RI), pulsatility index (UA PI), and systolic/diastolic ratio (SD ratio). Data were analyzed using independent sample t-tests, with p-values ≤ 0.05 considered statistically significant.
Results: Women with gestational hypertension had significantly higher UA RI (0.63 vs. 0.58, p = 0.013), UA PI (0.88 vs. 0.80, p < 0.001), and SD ratio (2.91 vs. 2.28, p < 0.001) compared to women without hypertension. Women with oligohydramnios had lower UA RI (0.57 vs. 0.62, p = 0.025) but higher UA PI (0.77 vs. 0.86, p < 0.001) and SD ratio (2.34 vs. 2.68, p < 0.001) compared to women without. Among women with both conditions, UA PI (0.89 vs. 0.82, p = 0.002) and SD ratio (2.93 vs. 2.47, p < 0.001) were significantly elevated.
Conclusion: Gestational hypertension is associated with increased UA RI, UA PI, and SD ratio, indicating higher vascular resistance. Oligohydramnios is characterized by lower UA RI but higher UA PI and SD ratio.
Keywords: Hypertension, Gestational hypertension, Oligohydramnios
Introduction
In the developed world, hypertensive disorders during pregnancy are the second-most common cause of direct maternal death [1]. The majority of medical pregnancy complications, including gestational hypertension, affect 5- 10% of pregnancies [2]. Black women, women over 45, and women with diabetes have the highest rates [3]. The published rates may understate current incidence given the rising prevalence of baseline diabetes, obesity, and hypertension in women of reproductive age as well as the trend towards older maternal age. Pregnancy-related hypertension is linked to an increased risk of intrauterine death, placental abruption, intrauterine growth restriction, preterm, and intracerebral hemorrhage. Pregnancy hypertension incidence was significantly lower in Pakistan (9.3%) than India (10.4%), Mozambique (11.0%), or Nigeria (10.1%). Most hypertension was diastolic only (46.5% India, 72.6% Pakistan, 61.1% Mozambique, and 63.2% Nigeria) [4].
Systolic blood pressure (BP) of 140 mmHg or higher and/or diastolic BP of 90 mmHg or higher on at least two occasions more than 6 hours apart when resting are diagnostic markers for hypertensive disorders of pregnancy [5]. Severe hypertension is often defined as a systolic blood pressure reading of 160 mmHg or higher and/or a diastolic blood pressure reading of 110 mmHg or higher taken on two separate occasions [6]. Yet, the likelihood of life-threatening eclamptic events is not correlated with the severity of hypertension [7].
Reduced amniotic fluid volume (AFV) for gestational age is referred to as oligohydramnios. At 34 to 36 weeks of gestation, the amniotic fluid volume (AFV) increases linearly until it reaches a plateau (about 400 mL), where it then stays constant until term [8]. Around 40 weeks of gestation, the AFV starts to gradually decline, which results in post-term gestations with less volume. This pattern enables ultrasonography analysis and fundal height measures to be used for clinical assessment of AFV throughout pregnancy [9].
Pregnancy problems and difficulties that are linked to oligohydramnios fall into the following categories: maternal, fetal, placental, and idiopathic. Any medical or obstetric disease that causes utero placental insufficiency (8% of all pregnancies) has an association with oligohydramnios [10]. Chronic hypertension, vascular disease, thrombophilia, and preeclampsia are a few potential causes. It also has a connection to several medications and maternal diabetes [10].
Using ultrasound Doppler, foetal hemodynamics can be measured non-invasively. Doppler studies of the umbilical arteries (UA) provide information on the fetoplacental circulation’s perfusion as well as the specific foetal organs that are useful in identifying hemodynamic abnormalities brought on by foetal hypoxia and anemia [11]. It has been discovered that oligohydramnios and gestational hypertension are both connected to IUGR and changed Doppler indices. Knowing exactly how one of these two risk factors interacts with the other when present either separately or concurrently to complicate feto-maternal outcomes is crucial.
Materials and methods
A case control study was conducted at Radiology Department of Indus Hospital, Lahore. The sample size was calculated at 95% level of confidence and 5% margin of error. The anticipated mean of resistive index of umbilical artery in four groups Normal, Oligohydramnios, hypertensive and Oligohydramnios with hypertensive were 0.64, 0.66, 0.68 and 0.75 [31] respectively.
88 pregnant women aged 18-35 years were enrolled in current study and further divided in 4 groups each consists of 22 women. Group I: oligohydramnios, Oligohydramnios is defined as decreased amniotic fluid volume (AFV) relative to gestational age. Transabdominal ultrasound evaluation of AFV includes the use of either the maximum vertical pocket (MVP). The normal range for MVP was 2-8 cm: a pocket <2cm was considered oligohydramnios [32]. Group II: gestational hypertension, Gestational Hypertension also referred to as Pregnancy-Induced Hypertension (PIH) is a condition characterized by high blood pressure during pregnancy. Women with Systolic and Diastolic blood pressure of 140/80 were taken as hypertensive [33]. Group III: oligohydramnios with gestational hypertension, Group IV: Normal. All women with gestational diabetes mellitus, prolapsed cord, IUGR, membrane rupture/ amniotic leakage and vaginal bleeding were excluded from study. Study was conducted after the approval from Research ethical committee, the University of Lahore REC-UOL-/189-07 /2022. Transabdominal Doppler examination was carried out with the patient in the supine position in both normal and hypertensive mothers in quiet respiration using 3-5 MHz curvilinear probe. Initially the umbilical blood vessels were recognized and the various indices recorded. Sampling site for the umbilical artery was mid cord level. Data was analyzed by statistical software for social sciences (SPSS version 25.0). Independent sample t-test was applied. P-value less or equal 0.05 was considered significant.
Results
The mean age was 26.5 ± 3.1 years. The mean gestational age was 36.6 ± 1.7 weeks. The mean amniotic fluid index was 8.3 ± 4.4 cm. For blood pressure, the average systolic reading is 124.5 ± 5.9 mmHg, and the average diastolic reading is 85.1 ± 5.0 mmHg. In terms of parity, 37 women have had no previous births, 23 have had one previous birth, 16 have had two, 7 have had three, 3 have had four, and 1 woman each has had five and six previous births, respectively. 44 women (50%) have gestational hypertension, while the other 44 (50%) do not. Concerning gestational hypertension, 20 women (22.7%) are affected, whereas 68 women (77.3%) are not. For oligohydramnios, 23 women (26.1%) have this condition, and 65 women (73.9%) do not. Finally, 24 women (27.3%) have both oligohydramnios and gestational hypertension, while 64 women (72.7%) do not have both conditions (Table 1).
Women with gestational hypertension have higher UA RI (0.63 vs. 0.58, p = 0.013. The UA PI (Umbilical Artery Pulsatility Index) for hypertensive women was 0.88 ± 0.09, while it is 0.80 ± 0.08 for non-hypertensive women, showing a highly significant p-value of 0.000. The SD ratio (Systolic/Diastolic) is 2.91 ± 0.20 for hypertensive women and 2.28 ± 0.20 for non-hypertensive women, with a p-value of 0.000. Regarding oligohydramnios, women with this condition have a UA RI of 0.57 ± 0.10, whereas those without have a UA RI of 0.62 ± 0.08, with a p-value of 0.025. The UA PI is 0.77 ± 0.09 for women with oligohydramnios and 0.86 ± 0.09 for those without, with a p-value of 0.000. The SD ratio is 2.34 ± 0.26 for those with oligohydramnios, compared to 2.68 ± 0.37 for those without, with a p-value of 0.000. For women with gestational hypertension, the UA RI is 0.64 ± 0.07, while it is 0.60 ± 0.09 for those without, with a p-value of 0.051. The UA PI for women with gestational hypertension is 0.87 ± 0.07, compared to 0.83 ± 0.10 for those without, with a non-significant p-value of 0.157. The SD ratio is significantly higher in women with gestational hypertension (2.83 ± 0.26) compared to those without (2.52 ± 0.37), with a p-value of 0.001. For the combination of oligohydramnios with gestational hypertension, the UA RI is 0.62 ± 0.11 for affected women and 0.60 ± 0.82 for those without, with a non-significant p-value of 0.354. The UA PI is 0.89 ± 0.10 for women with both conditions, compared to 0.82 ± 0.08 for those without, with a p-value of 0.002. The SD ratio is significantly higher at 2.93 ± 0.22 for those with both conditions, compared to 2.47 ± 0.34 for those without, with a p-value of 0.000 (Table 2).
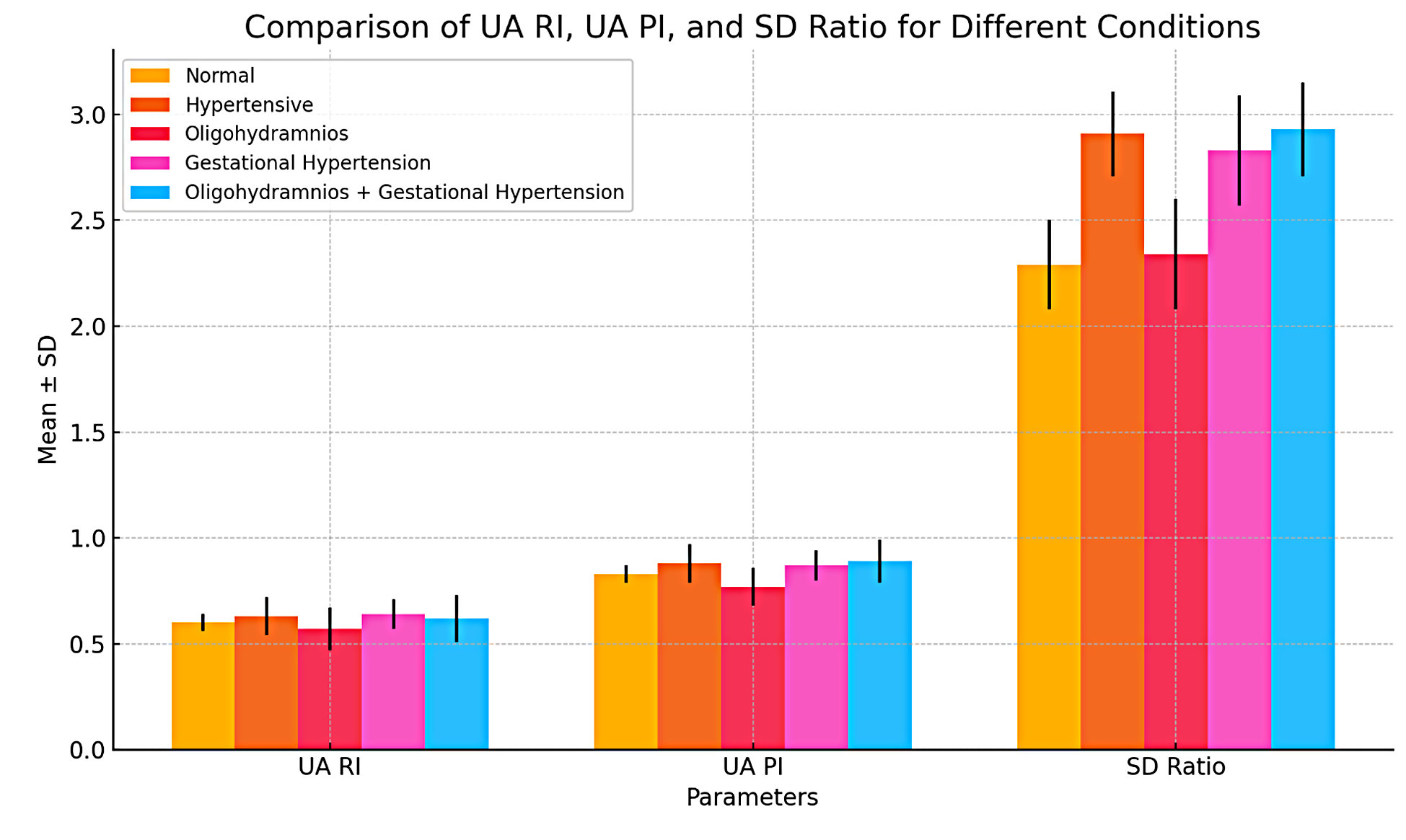
Figure 1. Comparison of umbilical artery Doppler between normal, hypertensive, oligohydramnios, gestational hypertension, and oligohydramnios with gestational hypertension.
Discussion
Many factors, including fetal urine volume, amniotic fluid perfusion, and maternal hydration, affect the amount of amniotic fluid [13]. The purpose of this study was to examine the hemodynamic changes in the umbilical artery in third-trimester pregnant women with oligohydramnios, gestational hypertension, and those who had both conditions. Oligohydramnios was found to be a very reliable predictor for these problems by Manning et al when they examined a sizable, pre-screened population of individuals [14]. The UA has evolved into the first and most extensively researched foetal artery since the use of ultrasonography Doppler in obstetrics. According to Tannirandorn and Phaosavasdi’s research, pregnancy complications including oligohydramnios and foetal hypoxia are frequently associated with the lack of diastolic flow [15,16].
In our study, the mean + SD maternal age was 26.5 + 3.1 years. In a study conducted by Jabeen et al. [17], the average age of the pregnant women was 33.67 years. Ayyuba et al. [18], also conducted a study on women age 31.33 ± 5.92 years. Maternal age in Rasool et al. [19], study was similar to our study as the mean age was 26.7 ± 4.1 years, the mean age of Akin et al [20] study population was also 25±4. The mean + SD gestational age in our study was 36.6 + 1.7 weeks, in study conducted by Jabeen et al [17] the average gestational period was 33.57 weeks. In Udo et al [21] study the gestation age was 26 and 40 weeks. In Rasool et al’s [19] study the gestational age was 32.1 ± 2.3 weeks, and in Akin et al [20] the average gestational age was 37.4±1 weeks.
In our study mean + SD umbilical artery Pulsatility index (PI) was 0.8 + 0.1, Resistive index (RI) was 0.6 + 0.09 and mean systolic to diastolic (S/D) ratio was 2.5 + 0.3 in all 88 participants. In Jabeen’s et al [17] study the mean± systolic to diastolic (S/D) ratio for the umbilical artery was 3.01 ± 0.79 SD. The mean ± SD pulsatility index (PI), of the umbilical artery, was 1.11 ± 0.64. The mean ± SD resistive index (RI) of the umbilical artery was 0.66± 0.13 SD.
In our study, the mean umbilical artery resistive index in normal women and women with oligohydramnios was 0.6. However, in women with gestational hypertension and in women with both gestational hypertension and oligohydramnios mean RI of umbilical artery was 0.7. In Rasool et al [19] study the mean ± SD resistivity Index in normotensive women was 0.6 ± 0.1 and in hypertensive women, RI was 0.7 ± 0.01. In Awan et al [22] study the umbilical artery RI for hypertensive women was 0.68 and in normotensive mean RI was 0.64. In Hashemi et al [23] study the mean umbilical artery RI was 0.77 in study group and 0.64 for control group. Esawi et al [24] stated in his study that resistive index of umbilical artery in pregnancy induced hypertension group is higher 0.63±0.11 than control group 0.62±0.06. Udo et al [21] research also indicated high RI in hypertensive group as compared to normal.
The mean umbilical artery pulsatility index in normal women and in women with oligohydramnios was 0.8. In women with gestational hypertension and in women with both gestational hypertension and oligohydramnios mean PI of umbilical artery was 0.9. The mean PI of umbilical artery in hypertensive group of Rasool et al [19] was 1.3 ± 0.4 and in normotensive group 1.0 ± 0.3. In Awan et al [22] study, Mean and standard deviations for the pulsatility index were 1.4 + 0.3 and 1.2 + 0.2 in hypertensive and normotensive. In Hashemi et al [23] study the calculated mean values of umbilical artery PI was 1.39 in hypertensive and 1.08 in normal controls. In study conducted by Esawi et al [24] the mean ±SD of PI of the umbilical artery was 0.97± 0.33 the PIH group and 0.91 ± 0.17 in control group. In study conducted by Udo et al [21] the mean Umbilical artery PI was 0.9 ± 0.2 and 0.8 ± 0.2 for the hypertensive and normal pregnancies respectively.
The mean umbilical artery S/D ratio in normal women and oligohydramnios was 2.3. In addition, in women with gestational hypertension and with both gestational hypertension and oligohydramnios mean S/D ratio of umbilical artery was 2.9. Rasool et al [19] hypertensive women as compared to normotensive women the mean systolic/diastolic flow was 3.8 ± 0.9 and 2.6 ± 0.5 respectively. In Awan et al [22] study the mean and standard deviations for the S/D ratio was 5.0 + 1.3 and 3.4 + 0.5 in hypertensive and normotensives respectively. Hashemi et al [23] the calculated mean values of spectral Doppler indices in the case group was 4.88 for S/D ratio while in the control group was calculated as 2.9. Esawi et al [24] conducted a study the mean ±SD of S/D of the umbilical artery was 2.9±1.38 in the PIH group and 2.72 ± 0.41 in control group. Udo et al [21] study revealed similar results with a higher mean S/D ratio in hypertensive and normal range in normotensive.
Our findings, which are consistent with a research by Rehab et al., [25] show that the Doppler indices of both umbilical arteries in the control group decline with increasing gestational age and Khalid, et al., [26] found all Doppler indices exhibited a steady drop with increasing gestational age at normal pregnancy. In our study, normotensive mothers umbilical artery Doppler indices are in the normal range, but those of gestational hypertension mothers are higher. In a study conducted by Rehab et al [25], the mean of the umbilical artery’s PI and RI were markedly greater in the high-risk group than in the control group. Our findings are almost identical to those of the research by Bansal et al. [27] reported that the umbilical artery’s PI, RI, and S/D were considerably higher in the study group than in the control group.
Interestingly, women with oligohydramnios demonstrated lower UA RI but higher UA PI and SD ratios compared to women without oligohydramnios. This finding is noteworthy because it suggests that reduced amniotic fluid volume may affect placental perfusion and fetal oxygenation, which are critical to fetal well-being. Lower UA RI typically reflects less resistance to blood flow, which is usually a positive sign, but in the context of oligohydramnios, it may also signal abnormal placental hemodynamics, leading to increased PI and SD ratios. The higher PI and SD ratios observed in these women suggest compromised blood flow and may indicate the presence of fetal distress or growth restrictions due to the altered perfusion [34].
The findings of this study are consistent with previous studies that reported a significant difference in fetal growth parameters among different groups of pregnant women, including those with gestational hypertension, preeclampsia, and fetal growth restriction [28, 29]. Furthermore, a previous study reported that the estimated fetal weight was lower in women with oligohydramnios and hypertensive disorders compared to women with normal amniotic fluid and normotensive women [30].
While these findings are consistent with prior research, it is important to interpret them in light of certain limitations. The study was conducted at a single center, which may limit the generalizability of the results. Furthermore, other factors such as maternal comorbidities, medication use, and lifestyle factors were not comprehensively controlled, which could have influenced the outcomes.
The clinical significance of these findings lies in the ability to use umbilical artery Doppler indices as predictive markers for adverse pregnancy outcomes in high-risk pregnancies. Monitoring these indices can help in early detection of fetal growth restriction and hypoxia, which may prompt timely interventions.
For future research, it would be beneficial to explore the underlying mechanisms that cause the distinct changes in the Doppler indices observed in women with oligohydramnios and gestational hypertension. Additionally, studies involving larger, multi-center cohorts would help validate these findings and determine the optimal cut-off values for Doppler indices in predicting fetal distress.
Conclusion
In conclusion, women with gestational hypertension tend to have higher readings in UA RI, UA PI, and SD ratio, indicating increased resistance and altered blood flow in their arteries. Similarly, women with oligohydramnios have lower UA RI but higher UA PI and SD ratios compared to those without this condition, suggesting potential issues with blood flow. These patterns are even more pronounced when both oligohydramnios and gestational hypertension are present, highlighting the increased risk and the need for careful monitoring. These results emphasize the importance of checking blood flow and resistance in managing high-risk pregnancies to ensure better health for both the mother and the baby. Monitoring of these fetal growth parameters can be useful in the clinical management of high-risk pregnancies and may help in the timely detection of fetal growth restriction and hypoxia.
Author’s contribution
R.M., M.Z; Data Collection, S.M.Y.F., S.A.G; Formal Analysis, Supervision, Writing – original draft, R.M Writing – review & editing.
Funding
None.
Study registration
N.A.
Disclosure of interests
The authors declare that they have no conflict of interests.
Ethical approval
This research was approved by the Research Ethical Committee of The University of Lahore (ethics code: REC-UOL-/189-07/2022).
Informed consent
All participants were provided with necessary explanations about the objectives and each enrolled patient gave informed consent to allow data collection and analysis for research purposes prior to the start of the study.
Data sharing
The datasets used and analyzed during the current study are available under reasonable request to the corresponding author.
References
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118 Suppl 1:1–203. doi: 10.1111/j.1471-0528.2010.02847.x.
- Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. doi: 10.1067/mob.2000.107928.
- Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, et al. Racial disparity in hypertensive disorders of pregnancy in New York state: a 10-year longitudinal population-based study. Am J Public Health. 2007;97(1):163–70. doi: 10.2105/AJPH.2005.068577.
- Magee LA, Nathan H, Adetoro OO, Bellad MB, Goudar SS, Lee T, et al. 256. The incidence of pregnancy hypertension in the Community Level Interventions for Pre-eclampsia (CLIP) trials–Population-level data from Mozambique, Nigeria, India and Pakistan. Pregnancy Hypertension. 2018 Oct 1;13:S36. doi: 10.1016/j.preghy.2018.08.107.
- Magee LA, Helewa M, Moutquin J-M, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(3 Suppl):S1–S48. doi: 10.1016/S1701-2163(16)32776-1.
- Redman CWG. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97(23):1967–9. doi: 10.1136/heartjnl-2011-300949.
- Regitz-Zagrosek V, Lundqvist CB, Borghi C, Cifkova R, Ferreira R, Foidart J-M, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy The Task Force on the Management of Cardiovascular Diseases During Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32: 3147–97. doi: 10.1253/circj.CJ-66-0035.
- Rabie N, Magann E, Steelman S, Ounpraseuth S. Oligohydramnios in complicated and uncomplicated pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017 Apr;49(4):442-449. doi: 10.1002/uog.15929.
- Magann EF, Bass JD, Chauhan SP, Young RA, Whitworth NS, Morrison JC. Amniotic fluid volume in normal singleton pregnancies. Obstet Gynecol. 1997 Oct;90(4 Pt 1):524-8. doi: 10.1016/S0029-7844(97)00351-7.
- Peipert JF, Donnenfeld AE. Oligohydramnios: a review. Obstet Gynecol Surv. 1991 Jun;46(6):325-39.
- Mohan S, Natarajan P, Madineni S, Rajasekhar K: Study of triple vessel wave pattern by Doppler studies in low risk and high risk pregnancies and perinatal outcome. J Obstet Gynaecol India. 2017;16:14–23. Doi: 10.9790/0853-1603061423.
- Hou L, Wang X, Hellerstein S, Zou L, Ruan Y, Zhang W. Delivery mode and perinatal outcomes after diagnosis of oligohydramnios at term in China. J Matern Fetal Neonatal Med. 2020 Jul;33(14):2408-2414. Doi: 10.1080/ 14767058.2018.1553944.
- Beall M, Van den Wijngaard J, Van Gemert M, Ross M: Regulation of amniotic fluid volume. Placenta. 2007;28(8–9):824–832. Doi: 10.1016/j.placenta.2006.12.004.
- Manning FA, Platt LD, Sipos L: Antepartum fetal evaluation: development of a fetal biophysical profile. Am J Obstet Gynecol. 1980;136(6):787–795. Doi: 10.1016/0002-9378(80)90457-3.
- Malik R, Saxena A: Role of colour Doppler indices in the diagnosis of intrauterine growth retardation in high-risk pregnancies. J Obstet Gynaecol India. 2013;63(1):37–44. Doi: 10.1007/ s13224-012-0210-4.
- Tannirandorn Y, Phaosavasdi S: Significance of an absent or reversed end-diastolic flow velocity in Doppler umbilical artery waveforms. J Med Assoc Thai. 1994;77(2):81–86.
- Jabeen Z, Bacha R, Zain-ul-Hassan, Fatima M, Manzoor I, Ramzan I, et al. Hemodynamic Changes in Umbilical Artery and Middle Cerebral Artery With Oligohydramnios in Third Trimester of Pregnancy. Journal of Diagnostic Medical Sonography. 2022 Mar;38(2):147-53. Doi: 10.1177/87564793211051978.
- Ayyuba R, Abubakar IS, Yakasai IA. Umbilical artery Doppler velocimetry study on prediction of adverse pregnancy outcomes among pregnant women with hypertensive disorders in Kano, Nigeria. Nigerian Journal of Basic and Clinical Sciences. 2015 Jul 1;12(2):95. Doi: 10.4103/0331-8540.169296.
- Rasool Z, Batool N, Ali A, Arshad N, Dar WM. The Evaluation of Doppler Indices for Umbilical Artery in Hypertensive Women in Third Trimester of pregnancy. Medical Science Journal for Advance Research. 2022 Jun 11;3(2):40-7. Doi: 10.46966/msjar.v3i2.39.
- Akin I, Uysal A, Sanci M, Kurtulmus S, Ispahi C, Uysal F, et al. Applicability of fetal renal artery Doppler values in determining pregnancy outcome and type of delivery in idiopathic oligohydramnios and polyhydramnios pregnancies. Ginekologia polska. 2013;84(11).
- Udo DU, Igbinedion BO, Akhigbe A, Enabudosoe E. Assessment of uterine and umbilical arteries Doppler indices in third trimester pregnancy-induced hypertension in UBTH, Benin-City. Nigerian Medical Practitioner. 2017 Jun 5;71(3-4):33-8.
- Awan MW. Comparison of Fetal Umbilical Artery Doppler Indices between Normal and Hypertensive Pregnant Women in the Second Trimester of Pregnancy. Ann. Pak. Inst. Med. Sci. 2015;11(2):95-9.
- Hashemi A.H., Naghibi S., Afzali Narges, Hashemi S.. Changes of Umbilical Artery Blood Flow Due to Pregnancy Induced Hypertension in the Third Trimester of Pregnancy. Iranian Journal of Radiology. 2010;7(Supplement 1 (26th Iranian Congress of Radiology)):0-0. Available from: https://sid.ir/paper/284063/en.
- Al Esawi R, Edan B, Shukur Z. Umbilical Artery Flow Velocity in Pregnancy Induced Hypertension at Third Trimester. Kufa Medical Journal. 2017;17(1):92-100. Doi: 10.36330/kmj.v17i1. 1952.
- REHAB MH, El-Sayed E, Marwa M. Doppler ultrasonography of foetal middle cerebral and umbilical arteries in high risk pregnancy. The Medical Journal of Cairo University. 2018 1;86:3451-8. Doi: 10.36330/kmj.v17i1.1952.
- Khalid M, Wahab S, Kumar V, Khalid S, Haroon S, Sabzposh NA. Doppler indices in prediction of fetal outcome in hypertensive pregnant women. Nepal Journal of Obstetrics and Gynaecology. 2011;6(1):28-34.
- Bansal A, Choudhary J, Gupta H. Role of panvessel Doppler study in high risk pregnancy. Diabetes. 2015;3:6.
- Maruotti GM, Saccone G, Martinelli P. Fetal growth restriction: current perspectives. J Prenat Med. 2012;6(3):56-9.
- Rizzo G, Capponi A, Pietrolucci ME, Arduini D. The role of Doppler in the management of high-risk pregnancies. Best Pract Res Clin Obstet Gynaecol. 2004;18(3):569-85.
- Combs CA, Garite T, Maurel K, Das A, Porto M. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol. 1990;162(5):1168-73. Doi: 10.1016/0002-9378(90)90009-V.
- Gosling R, King D: Ultrasound angiology. Arteries and Veins 1975;1:61–71.
- Luton D, Alran S, Fourchotte V, Sibony O, Oury J-F: Paris heat wave and oligohydramnios. Am J Obstet Gynecol 2004;191(6):2103–2105.
- Simanaviciute D, Gudmundsson S: Fetal middle cerebral to uterine artery pulsatility index ratios in normal and pre-eclamptic pregnancies. Ultrasound Obstet Gynecol. 2006;28(6):794–801.
- Selam B, Koksal R, Ozcan T. Fetal arterial and venous Doppler parameters in the interpretation of oligohydramnios in postterm pregnancies. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 May;15(5):403-6.