Research
HJOG 2022, 21 (1), 35-46 | doi: 10.33574/hjog.0404
Aloy Ugwu, Ayodeji Oluwole, Kehinde Okunade, Sarah John-Olabode, Nneoma Ani-Ugwu, Olayemi Olumakinwa, Amaobichukwu Anyanwu, Emmanuel Ugwu, Christian Makwe
Department of Obstetrics and Gynaecology, Lagos University Teaching Hospital, Lagos, Nigeria
Correspondence: Aloy Okechukwu Ugwu, Senior Registrar, Department of Obstetrics and Gynaecology, Lagos University Teaching Hospital, P.M.B. 12003, Surulere Lagos, Nigeria, e-mail: okeyugwu92@gmail.com, Tel.: +2347033132189
Abstract
Background: Preeclampsia (PE) is a multi-systemic progressive disorder that is unique to human pregnancy, occurring usually after 20 weeks of gestation. Useful biochemical markers to be utilized for early prediction of preeclampsia continue to elude us. The search for biomarkers is aimed identifying women at increased risk of developing PE, so that medical interventions may be instituted very early in pregnancy to ameliorate its occurrence and improve maternal and fetal outcome.
Aim: This study aimed to determine the association between serum levels of Cystatin C and/or free β-subunit of hCG measured in early pregnancy and the development of pre-eclampsia and its severity.
Methods: This was a prospective cohort study of 290 pregnant women recruited during routine antenatal care, at the gestational age of between 13 and 19 weeks. After obtaining an informed consent from each participant, a structured questionnaire was used to collection relevant information followed by the collection of 5 mL of venous blood sample. Serum cystatin C and free sub unit of β-hCG levels were determined by standard enzyme-linked immunosorbent assay (ELISA) method. All participants were followed up till delivery and those who developed preeclampsia was classified as mild and severe preeclampsia.
Data were entered and analyzed using STATA version 16 statistical software. Hypothesis testing was done using chi-square test for categorical variables, and the independent-samples t-test and ANOVA for numerical variables.
Results: We found significantly elevated serum levels of cystatin C and free subunit of β-hCG in women who developed preeclampsia, (p < 0.002 and 0.001) respectively.
Conclusion: Our study has shown that in a healthy population of pregnant women that elevated serum levels of cystatin C and free beta hCG concentration measured in early second trimester were associated with an increased risk of developing preeclampsia and its severity. This suggests that the combination of these analytes may have a role as a marker of pre-eclampsia and its severity especially when used in combination.
Keywords: Cystatin C, early predictors, free β-hCG, hypertension, preeclampsia, severity
Introduction
Pre-eclampsia (PE) is a common medical disorder in pregnancy with various theories to explain its aetiopathogenesis1. It affects about 2-5% of pregnant women globally and contributes significantly to maternal and perinatal mortality and morbidity1,2,4. Although this disorder resolves spontaneously at various weeks postpartum, a sizeable proportion of these women may develop long term complications such as hypertension within a decade of an affected pregnancy, they may also be at risk of other cardiovascular diseases and type 2 diabetes mellitus1,3,5.
The most plausible theory of its onset is that of defective trophoblastic invasion and maternal response to endothelial dysfunction and disproportion of antiangiogenic and angiogenic biomarkers leading to clinical manifestation of the disease1,2,3. There have been several research works in attempt to identify distinctive screening biomarker that would predict the onset of preeclampsia prior to its clinical manifestation. Theoretically, these biomarkers when identified will help detect high-risk pregnancies and provide clinicians with additional tools for the prevention and treatment of preeclampsia6. Recently, maternal risk factors, placental growth factor, serum cystatin C, free beta hCG, mean arterial blood pressure, uterine artery pulsaltility index have been utilized for predicting PE, unfortunately, none of these can on its own predict the occurrence of PE. to be deployed to clinical use; a combination of two or more independent biomarkers, each reflecting a different pathophysiological process, may most likely be useful in developing a suitable predictive algorithm2,6.
The kidneys have an indispensable role in maternal physiological changes in pregnancy and in the pathophysiology of preeclampsia. Cystatin C, a cysteine protease inhibitor, have been projected to be a better marker of glomerular filtration; imbalance between serum cystatin C and cysteine proteases (cathepsins) have been implicated in defective trophoblastic invasion leading to development of preeclampsia7,8.
The production of human chorionic gonadotropin by the placenta in early pregnancy is critical for implantation and maintenance of the feto-placental unit9,10. It has been hypothesized that placental vascular damage and ischaemia might result in increased production of beta human chorionic gonadotropin (hCG) by hyperplasic cytotrophoblastic cells9,10,11, this may suggest a relationship between its secretion and development of PE.
Previous studies, by Thilaganathan et al. and Padma et al., have demonstrated elevated maternal serum cystatin in pregnancies complicated by PE7,12. In addition, Kanika et al., found that maternal serum level of β-hCG was markedly raised in women with preeclampsia, this finding was consistent with the report of Begum et al11.
This study therefore aimed to determine the predictive significance of combined early-pregnancy serum levels of cystatin C and free β subunit of hCG in the occurrence and severity of pre-eclampsia. This is because, these two biomarkers reflect different pathophysiological process in the development of PE.
Materials and Methods
This was a hospital based prospective cohort study carried out at the antenatal clinics of the Lagos University Teaching Hospital (LUTH) (a tertiary hospital) after obtaining ethical approval from the Health Research and Ethics Committee of the institution ADM/DCST/HREC/APP/2291.
The participants included healthy normotensive pregnant women between 13-19 completed weeks gestational age) at their booking visit at LUTH. Preeclampsia was defined based on International Society for the Study of Hypertension in pregnancy (ISSHP) classification.13,14 It Excluded from the study were pregnant woman with chronic hypertension, chronic infection, such as hepatitis or HIV or chronic kidney or cardiac disease; or women already using prophylactic acetylsalicylate (aspirin) treatment or assessed as high risk for PE. The primary outcome of the study was preeclampsia, while secondary outcome was severity of preeclampsia.
A structured and pre-tested interviewer-administered questionnaire was used to collect relevant information from each eligible participant. Venous samples were obtained from the participants under aseptic condition via venipuncture and these were subsequently assayed enzymatically with the ELISA (enzyme-linked immunosorbent assay) for the measurement of serum cystatin C and free sub unit of β-hCG. All the participants were followed up till delivery and were classified as mild, severe and non-PE.
Data were entered and analyzed using STATA version 16 statistical software (produced by StataCorp LLC USA and released in 2019). Data were summarized by descriptive statistics such as mean, and standard deviation, or median and interquartile range for numerical variables. Frequencies and percentages were utilized for categorical variables. Quantitative data were tested for normality with the Kolmogorov–Smirnov test. Associations between continuous variables and the binary outcomes (preeclampsia versus no preeclampsia) were tested using the independent sample t-test (normal distribution). Analysis of variance ANOVA test was used to assess the association between continuous variables and outcome with more than two (2) levels (normotensive, mild and severe preeclampsia). Whereas association between categorical variables and outcome was assessed using the chi-square (χ2) test or the Fisher exact test. Univariable and multivariable logistic regression modelling was conducted to determine the association between the predictor variables (serum Cystatin C, free sub unit of B-hCG) and the outcome variables (presence or absence of preeclampsia, and mild or severe preeclampsia). Receiver operating characteristics (ROC) curve were used to determine the predictive accuracy of early pregnancy serum Cystatin C only, free sub unit of B-hCG levels only and a combination of both markers in the occurrence and severity of preeclampsia. A Youden’s index was used to determine the optimal cut-off of the analytes for the prediction of preeclampsia. Two-tailed test of hypothesis was assumed. Statistically significant level was set at P-value <0.05.
The data sets were complete for 276 participants and were included in the statistical analysis (Figure 1). The demographic distribution of the participants is shown in Table 1. The mean maternal age of patients in our cohort was 32.5+6.5 years. Majority of the women had at least secondary education 194 (70.3%). Mean gestational age at enrollment was 16.2±2.7 weeks in women who developed preeclampsia and non-preeclampsia respectively. We found that 229 (83.0%) of the participants did not develop preeclampsia (178 (64.5) were normotensive while 51 (18.5%) women developed gestational hypertension), while 41(17.0%) of the women developed preeclampsia.
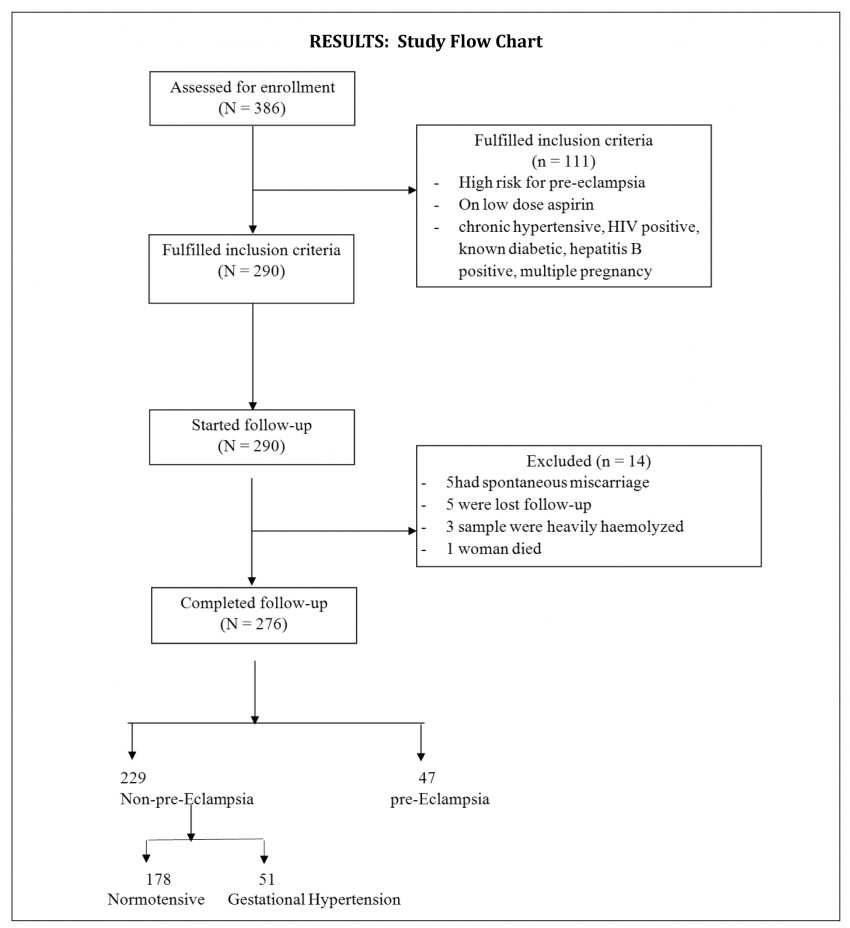
Figure 1. Participant flow chart
Table 2 shows the serum cystatin C and free β-hCG in participants who developed preeclampsia and those without preeclampsia. There was almost three -fold increase in mean maternal serum cystatin C levels among Preeclamptics as compared to those without preeclampsia (1.99mg/dl±0.24 vs 0.67mg/dl±0.04; P-value < 0.002). Similarly, there was significant increase in the mean serum free beta hCG in preeclamptic participants compared to those without preeclampsia p-value of 0.001.
Table 3: shows association between serum cystatin C, B-hCG and severity of preeclampsia among the study participants. The level of serum cystatin and free beta hcg were significantly higher in those that developed severe preeclampsia compared to the participants with mild preeclampsia, p-value 0.001.
In Figure 2 and Table 4, we compared the ROC curves of Cystatin and free β-hCG as predictors of preeclampsia. The area under the curve of the ROC was 95.3% (AUC = 0.9533) for free β-hCG and 85.4% (AUC = 0.8541) for cystatin C which showed that our model of the relationship between the analytes and preeclampsia had a discriminatory value of 95.3% and 85.4% in predicting preeclampsia for free β-hCG and cystatin C respectively. Furthermore, there was a statistically significant difference in the predictive value of Cystatin as compared to free β-hCG for predicting preeclampsia (P-value = 0.0002). (Figure 2 and Table 4)
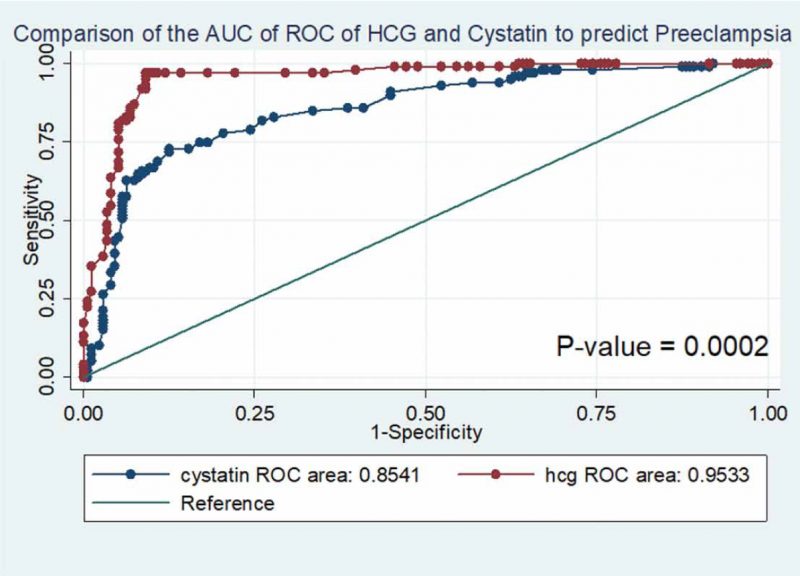
Figure 2. Comparison of the ROC curves of Cystatin and HCG as a predictor of Preeclampsia.
The normal range (5th to 95th percentile) of cystatin C among apparently normal pregnant women in our study was 0.32mg/dl – 2.3mg/dl. The optimum cut-off of serum cystatin was 1.8mg/dl. The sensitivity and specificity of diagnosing preeclampsia using serum cystatin C at cut-off of 1.8mg/dl was 65.5% and 82.4% respectively, the normal range (5th to 95th percentile) of free beta hCG among apparently normal pregnant women in our study was 152- 338iu/l. The optimum cut-off of free beta hCG was 235iu/l. The sensitivity and specificity of diagnosing preeclampsia using serum free β-hCG at cut-off of 235iu/l was 69.5% and 89.2% respectively.
After univariable regression modelling, elevated serum levels of the analytes (cystatin C and free β-hCG) were statistically associated with the development of preeclampsia (P-value <0.001). It was shown after multivariable regression modelling that included both free β-hCG and cystatin C, that women with high free β-hCG level (≥ 235iu/L) had about 2.9 folds odds of developing preeclampsia when compared to women with normal free β-hCG level (≤ 235) (AdjOR = 2.92, 95%CI: 4.87 – 6.85, P-value < 0.001); while women with high serum cystatin C level (≥1.8mg/dl) had about 7-fold higher odds of developing preeclampsia when compared to women with normal cystatin C level (< 1.8mg /dl) (AdjOR = 6.84, 95%CI: 2.3 – 20.36, P-value = 0.001) (Table 5).
In Table 6, its shown that there is steady increase of all the analytes from non-preeclampsia, mild and severe preeclampsia. It also shows that Most of the women who developed preeclampsia had severe preeclampsia (n= 37/47= 78.7%).
However, from Table 7 and Table 8, the post hoc Bonferroni test showed that there was a statistically significant difference in serum cystatin C level between women with mild preeclampsia and severe preeclampsia as well as non-preeclamptic participants (1.41±0.48 vs 1.95±0.29 vs 0.67 ± 0.04, P-value <0.003). Also, there was a statistically significant difference in serum cystatin C and free beta hCG levels in women with Severe preeclampsia as well as mild preeclampsia. participants. (189.09±20.16 vs 233.43±15.16 vs 178.28±5.9, P-value <0.001). Also, there was a statistically significant difference in serum free β-hCG level in women with Severe preeclampsia as well as mild preeclampsia.
Figure 3 shows that when we combine the two analytes the likelihood of predicting preeclampsia 96.7%. (Area under the ROC CURVE = 0.9671).

Figure 3. Receiver operator characteristic curve of the model combining both HCG and cystatin levels in predicting preeclampsia.
Discussion
The findings of this study suggest that there may be an association between the serum levels of cystatin C and free β-hCG in the occurrence and severity of preeclampsia in Nigerian women. The widespread endothelial dysfunction implicated in the pathogenesis of preeclampsia results in reduced vascular compliance and vasoconstriction resulting in hypertension. Although hypertension is a secondary sign of the disease, it is a very important sign because not only is it a therapeutic target in the management of preeclampsia, it is also an early indication of the disease.
We aimed to determine the predictive accuracy of serum levels of Cystatin C and/or free subunit of β-hCG in the occurrence and severity of preeclampsia among women in Lagos, Nigeria. It was shown that elevated maternal serum concentrations of cystatin C and free subunit of β-hCG were significantly associated with an increased risk of developing preeclampsia several weeks later. There was almost three -fold increase in mean maternal serum cystatin C levels among women who developed Preeclampsia when compared to those without preeclampsia (1.99mg/dl±0.24 vs 0.67mg/dl±0.04; P-value < 0.002). This elevated serum level of cystatin C agrees with the finding of 1.43 ± 0.24 mg/L in women with preeclampsia in a study by Wattanavaekin et al15.
In their study, the average serum Cystatin C level in pre-eclamptic patients was 1.43 ± 0.24 mg/L which is significantly higher than in normal pregnancy. This is also similar to the study by Risch et al8 where it was also found that serum cystatin C concentration measured in early pregnancy was significantly higher in women who subsequently developed preeclampsia (median 0.65 mg/L, interquartile range 0.59 to 0.75 mg/L) than in women who were normotensive at delivery (median 0.57 mg/L, interquartile range 0.50 to 0.63 mg/L)8. The similarity in this occurrence may be that their samples and ours were taken in early pregnancy with a mean gestational age of 14.2 weeks and 16 weeks respectively. This finding also favourably compares to that of Thilaganathan et al7 who also found that early pregnancy serum cystatin C level was significantly higher (p value <0001) in women with preeclampsia (median 0.65 mg/L, interquartile range 0.59 to 0.75 mg/L) than in women who did not develop preeclampsia (median 0.57 mg/L, interquartile range 0.50 to 0.63 mg/L). This finding may “support the hypothesis that the balance between trophoblast protease production and decidual protease inhibitor activity may have an important biologic role in trophoblast development and that a derangement in this balance may predispose to poor trophoblast development and the subsequent development of preeclampsia”7.
With regards to free β-hCG, our finding of elevated free β-hCG in women who developed PE is in agreement with earlier similar studies done by Kanika et al9. Their study also revealed significantly elevated serum level of maternal free β-hCG in women who preeclampsia. However, our finding is slightly different from that of Spencer et al16 whose study overall, revealed elevated serum level free beta hCG, although, not statistically significant. Our finding also corroborates the findings of Barjaktarovic et al17 where pregnant women with high serum level of free beta hCG concentration was associated with 1.5–2.7-fold increased risk of developing pre-eclampsia (P = 0.0001) in a population-based prospective cohort study. This finding of “elevated free subunit of β-hCG level in our study may suggest that the balance between pro- and anti-angiogenic factors during pregnancy may have a role in the pathophysiology of pre-eclampsia”16,17 Alternatively, our study differs from that of Ong et al18 where it was found that Low maternal serum beta hCG at 10-14 weeks of gestation are associated with subsequent development of pregnancy complications especially in those that developed preeclampsia, gestational hypertension and diabetes mellitus. The difference in our finding may be attributed to the population studied, our study consisted only of black women, also their inclusion and exclusion criteria was not specified.
In a retrospective cohort study by Goetzinger et al19, they demonstrated no statistically significant association between free β-hCG and pre-eclampsia across all defined thresholds. This difference may be attributed to the fact all their samples were taken in the first trimester whereas ours involved women in both first and second trimester. The reason for these contrasting findings by these authors may also be because of the differences in the hCG isoform measured, this may explain why some revealed elevated serum beta hCG and preeclampsia, others showed association between low level of beta hCG and preeclampsia or even no relationship.
We also compared the Receiver operating curves (ROC) of free subunit of β-hCG and cystatin C as predictors of preeclampsia. The predictive accuracy of the ROC showed that 95 and 85 out of one hundred women who had elevated serum levels of free subunit of β-hCG and cystatin C are most likely to develop preeclampsia, while the combination of these analytes was able to predict preeclampsia in 96 out of one hundred women. There was also a statistically significant difference in the predictive value of Cystatin as compared to free β-hCG for predicting preeclampsia (P-value = 0.0002).
The finding in our study is that serum cystatin C levels and free β-hCG were significantly elevated several months before women develop preeclampsia. These supports the hypothesis that a first trimester cathepsin/cystatin C imbalance may be implicated in the etiology of preeclampsia9,10,11. and that placental vascular damage leading to decreased oxygen supply and abnormal placental secretary function in patients with preeclampsia might result in increased hCG production by hyperplasic cytotrophoblastic cells9,10,11,19,20. However it also raises question about the possibility that cystatin C and free β-hCG may be biologically plausible and potentially useful early pregnancy markers for preeclampsia.
We were limited by the fact that only a single measurement of serum levels of cystatin C and free beta hCG were available for each woman rendering us unable to examine inter-individual differences in the analytes variation during pregnancy.
Our study has shown that in a healthy population of pregnant women that elevated serum levels of cystatin C and free beta hCG concentration measured in early second trimester were associated with an increased risk of developing preeclampsia and its severity. This suggests that the combination of these analytes may have a role as a marker of pre-eclampsia and its severity especially when used in combination.
Acknowledgments
We appreciate all our pregnant women who consented to participate in this study. We are also grateful to our head of department who allowed the conduct of this research under her leadership.
Disclosure
None declared.
References
1. Osanyin GE, Okunade KS, Ayotunde Oluwole A. Association between serum CA125 levels in preeclampsia and its severity among women in Lagos, South-West Nigeria. Hypertens Pregnancy. 2018 May;37(2):93-97.
2. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H. et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. International Journal of Gynecology & Obstetrics. 2019: 145(S1); 1–33.
3. Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013; 170:1-14.
4. Babah OA, Owie E, Ohazurike EO, Akinajo OR. Prevalence and pattern of medical disorders in pregnancy at the time of delivery at Lagos University Teaching Hospital, Lagos, Nigeria. Sub-Saharan Afr J Med 2018; 5: 93-98.
5. Babah OA, Olaleye O, Afolabi BB. Postpartum sequelae of the hypertensive diseases of pregnancy: A pilot study. Niger Med J 2018; 59: 1-6.
6. Park HJ, Shim SS, Cha DH. Combined Screening for Early Detection of Pre-Eclampsia. Int J Mol Sci. 2015 Aug 4;16(8):17952-74.
7. Thilaganathan B, Ralph E, Papageorghiou AT, Melchiorre K, Sheldon J. Raised maternal serum cystatin C: an early pregnancy marker for preeclampsia. Reprod Sci. 2009 Aug;16(8):788-93.
8. Risch M, Purde MT, Baumann M, Mohaupt M, Mosimann B, Renz H, Raio L, Surbek D, Risch L. High first-trimester maternal blood cystatin C levels despite normal serum creatinine predict pre-eclampsia in singleton pregnancies. Scand J Clin Lab Invest. 2017 Dec; 77(8):634-643.
9. Kanika MC, Munmun D, Sulekha G, Debasis B, Tapan KG. Value of Serum β-hCG in Pathogenesis of Pre-Eclampsia. J Clin Gynecol Obstet. 2012; 1(4-5):71- 75.
10. Wright A, Guerra L, Pellegrino M, Wright D, Nicolaides KH. Maternal serum PAPP-A and free β-hCG at 12, 22 and 32 weeks‘ gestation in screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2016 Jun;47(6):762-7.
11. Begum Z, Ara I, Tanira S, Keya Ka. The Association between Serum Beta human Chorionic Gonadotropin and Preeclampsia. J Dhaka Med Coll. 2014; 23(1): 89-93.
12. Padma Y, Aparna VB, Kalpana B, Ritika V, Sudhakar PR. Renal markers in normal and hypertensive disorders of pregnancy in Indian women: a pilot study. Int J Reprod Contracept Obstet Gynecol. 2013;2(4):514-520.
13. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97-104.
14. Hypertension in pregnancy: NICE guideline. Published 25 June 2019.
15. Wattanavaekin K, Kitporntheranunt M, Kreepala C. Cystatin C as a novel predictor of preterm labor in severe preeclampsia. Kidney Res Clin Pract. 2018 Dec;37(4):338-346.
16. Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005 Oct;25(10):949-53.
17. Barjaktarovic M, Korevaar TIM, Jaddoe VWV, de Rijke YB, Peeters RP, Steegers EAP. Human chorionic gonadotropin and risk of pre-eclampsia: prospective population-based cohort study. Ultrasound Obstet Gynecol. 2019 Oct;54(4):477-483.
18. Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000 Oct;107(10):1265-70.
19. Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. Predicting the risk of pre-eclampsia between 11 and 13 weeks’ gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat Diagn. 2010 Dec;30(12-13):1138-42.
20. Murmu S, Dwivedi J. Second-Trimester Maternal Serum Beta-Human Chorionic Gonadotropin and Lipid Profile as a Predictor of Gestational Hypertension, Preeclampsia, and Eclampsia: A Prospective Observational Study. Int J Appl Basic Med Res. 2020 Jan-Mar;10(1):49-53.