Research
HJOG 2022, 21 (1), 15-24| doi: 10.33574/hjog.0402
Sofoklis Stavros1*, Despoina Mavrogianni1, Lamprini Ntetsika1, Peter Drakakis1,2
1Molecular Biology of Reproduction Unit and Recurrent Abortions Unit, Assisted Reproduction Unit,
1st Department of Obstetrics and Gynecology, School of Medicine, Alexandra General Hospital of Athens, National and Kapodistrian University of Athens, Athens, Greece
2Third Department of Obstetrics and Gynecology, National and Kapodistrian University of Athens Medical School, Attikon Hospital, Athens, Greece
*This Research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers – 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (IKY)
Correspondence: Sofoklis Stavros, MD PhD, Obstetrician and Gynecologist, Fellow of the 1st Department of Obstetrics and Gynecology, National and Kapodistrian University of Athens, General Hospital of Athens ‘ALEXANDRA’, Lourou and Vasilissis Sofias Ave, 11528 Athens, Greece, Tel.: +306906725017, e-mail: sfstavrou@yahoo.com
Abstract
Introduction: Recurrent pregnancy loss (RPL) is defined as the spontaneous loss of two or more idiopathic consecutive clinical pregnancies prior to 22 completed weeks of gestation. Association of miRNA polymorphisms with RPL has been investigated as a genetic determinant for the risk of idiopathic RPL. Moreover, mutations in several interleukins’ genes have been also related to RPL.
Material and Methods: The study was carried out between September 2019 and September 2021 in Alexandra Maternity Hospital. Two hundred women with at least two consecutive spontaneous abortions (RPL) and 200 women as a control group who have completed one pregnancy were included in this study. Blood samples were collected from which genomic DNA was extracted, and PCR was performed to identify the IL1B -511T>C, IL6 -634G>C, IL-6 -174G > C, miR -149 T>C AND miR-27aA>G genotypes.
Results: Of the 5 genes tested, genotype and group correlation were found in IL-1b-511 and miR-27. In specific, in the IL-1b-511 gene, cases have a 77.2% statistically significantly higher relative probability of having the polymorphism (CC genotype) than the control group (OR = 1.772, p = 0.007). In addition, in the miR-27 gene, cases are 3.2 times statistically significantly more likely to have polymorphism (GG) compared to controls (OR = 3.283, p <0.001).
Conclusions: A genetic panel related to recurrent abortions is proposed. Gynecologists and researchers may apply it to establish a personalized treatment in women with RPL. The establishment of genetic panels related to infertility issues leads to more efficient solutions for the achievement of a pregnancy.
Keywords: IL1B -511T>C, IL6 -634G>C, IL-6 -174G > C, miR -149 T>C ,miR-27aA>G, polymorphisms, RPL
Introduction
Recurrent pregnancy loss (RPL) is defined as the spontaneous loss of two or more idiopathic consecutive clinical pregnancies prior to 22 completed weeks of gestation according to American Society of Reproductive Medicine (ASRM), European Society of Human Reproduction and Embryology (ESHRE), and several medical societies guidelines1,2. Due to its vast and its poorly defined etiology though, it remains a great challenge of reproductive medicine. In addition, 1–5% of couples trying to conceive may be suffer a recurrent pregnancy loss3.
Although the underlying cause of RPL is often unknown, a battery of environmental and biological causes has been related with increased incidence of RPL4. Chromosomal abnormalities, uterine anatomical anomalies, endocrine dysfunction and maternal infections, inherited thrombophilia have all been implicated as etiologic factors5-8, including genetic variants9,10.
Posttranscriptional target genes regulation is solid associated with microRNAs. Maintenance of stem cell-ness and modulation of differentiation are imperative regulatory functions served by microRNAs. MiRNAs are short, single stranded RNA molecules of ∼ 22 nucleotide (20 to 24 nucleotides); they are also noncoding small regulatory RNAs locating into the RNA induced silencing complex, recognizing the 3-untranslated region (3’-UTR) of target mRNAs, and thereby downregulate the gene expression through RNA silencing mechanisms, including translational inhibition and mRNA degradation11. More than 60% of all human protein-coding transcripts have been estimated that are under control of miRNAs12. Hence, a wide range of biological processes including cell differentiation, cell cycle progression, and programmed cell death is subject to miRNA-dependent regulation13. In addition, it is notable that miRNAs play pivotal and critical roles, implicating in processes related to the embryo implantation, embryo-maternal communication, pregnancy establishment, and embryo development14-16.
Recently, the association of miR-149 T > C (rs2292832), polymorphisms with RPL has also been investigated as a genetic determinant for the risk of idiopathic RPL17. In addition, a recent study showed an association between the miR-27a variant G allele and a lower RPL risk18.
Moreover, mutations in several interleukins’ genes have been also related to RPL; these interleukins are implicated to different functions such as the regulation of inflammatory and immune processes. Polymorphisms in interleukins (IL-1A, IL1B, IL-6, IL-18 and IL-10) are associated with increased risk of RPL based on previous epidemiological studies19.
In specific, interleukin 6 (IL6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. The human IL-6 gene is located to chromosome 7p21-24, consists of five exons, four introns, and a proximal promoter region of 303 bp19. In 2019, a recent metanalysis by Salimi and colleagues revealed that the IL-6 -174 G > C, -634 G > C polymorphisms might contribute to the susceptibility of RPL20.
Interleukin-1 (IL1) is a proinflammatory cytokine with multiple biological consequences, stimulating several events, such as B cell maturation and proliferation, helper T cell costimulation, and NK cell activation21. A recent study by Kim end colleagues in 2014, confirmed that women with a history of RPL had a higher proportion or total number of CD16 CD56 NK cells in the peripheral blood4.
Three proteins (IL1α, IL1β, and their naturally occurring inhibitor IL1RN) are encoded by IL1 gene family mapped on chromosome 2q13-14. lL1β protein is produced by Interleukin1β (IL1β, rs16944) gene; that protein is a proinflammatory cytokine implicated in both acute and chronic inflammation. A transition from C to T (in the minus strand) or in G to A (in the plus strand) promoter region at − 511 position has been associated with increased transcriptional activity4,22.
Material and Methods
Study Participants
The study was carried out between September 2019 and September 2021 in Alexandra Maternity Hospital. Two hundred women with at least two consecutive spontaneous abortions (RPL) and 200 women as a control group who have completed one pregnancy were included in this study. RPL group (n=200) with an age range between 25-40 were compared with the control group (n=200) formed from healthy singleton pregnancies, without any pregnancy complications. The study was approved by the local ethical committee in our hospital and informed consent was obtained from all participants. This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers – 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ)
Genotyping
Genetic material (DNA) was extracted from peripheral blood of women with RPL and controls, respectively, using the Monarch Genomic DNA Purification kit (New England Biolabs). Il-6 634G>C, Il-6 174G>C, Il1β511T>C, miR149(rs2292832), miR27a (rs895819) polymorphisms were genotyped in both groups performing Polymerase Chain Reaction(PCR) with the use of New England Biolabs Taq Polymerase and Restriction Fragment Length Polymorphism analysis (RFLP). The primers for each Single Nucleotide Polyrosphism were as follows:IL-1β(-511T>C) F5’TGGCATTGATCTGGTTCATC3’,R 5’GTTTAGGAATCTTCCCACTT3’ Restriction enzyme:AvaI)23,24 IL-6 (-634 C>G) F5’GAGAGGCCTTGAAGTAACTG3’,R5’AACCAAAGATGTTCTGAACTGA3’ Restriction enzyme:BsrBI)23,24 IL-6 (-174G>C) F, 5’GGAGTCACACACTCCACCT3’,R 5’GTGGGGCTGATTGGAAACC3’ Restriction enzyme:SfaNI23-25, miR149 F 5′-TGTCTTCACTCCCGTGCTTGTCC-3′ R 5′-TGAGGCCCGAAACACCCGTA-3′ (Restriction enzyme PvuII)17, miR27a F 5’-GAACTTAGCCACTGTGAACACCACTTG G3’ R 5’-TTGCTTCCTGTCACAAATCACATT G-3’ Restrictionenzyme:DraIII18.
The PCR and RFLP products were analysed on 3% agarose gel. Statistical analysis was performed via chi-squared test.
Statistical analysis
Statistical methods
To check the difference in the distribution of genotypes in the two groups, the non-parametric Chi-square control and the Fisher’s exact test on a case-by-case basis (Fisher’s exact test) were used. Similarly, a Chi-square test was used to test the correlation between each other and the group. The SPSS version 20 software as well as the website of the Munich Institute of Human Genetics http://ihg.gsf.de were used for the organization and analysis of the data. The level of statistical significance was set at 5%.
Results
In the control group, the percentages of women homozygous for the wild type allele, , women heterozygous, IL-6-634CG and women homozygous for the IL6 -634G>C polymorphism, were 67,5%, 17%, and 15,5%, respectively. In the group of patients with recurrent spontaneous miscarriages, the percentages were 67,3 %, 18,1% and 14,6% respectively. There was no statistically significant difference between the two groups (Table 1, Figure 1). As far as the IL-6 -174G > C polymorphism is concerned, in the control group, 146 (73,0%) women were homozygous for the wild type allele, 48 (24,0%) women were heterozygous, and 6 (3,0%) women were homozygous for the IL-6 -174G > C polymorphism. In the group of patients with recurrent spontaneous miscarriages, the percentages were 65,8%, 28,6% and 5,5 % respectively (Table2, Figure 2). Similarly, no statistically significant difference between the two groups was observed. Interestingly, a statistically significant difference between the two groups was unraveled as for the IL1B -511T>C polymorphism. In specific, in the control group, 82 (41,0%) women were homozygous for the wild type allele, , 63 (31,5%) women were heterozygous, IL1B -511TC and 55 (27,5%) women were homozygous for the IL1B -511T>C polymorphism. In the group of patients with recurrent spontaneous miscarriages, the percentages were 30,7%, 29,1% and 40,2% respectively. In the IL-1b-511 gene, cases have a 77.2% statistically significantly higher relative probability of having the polymorphism (CC genotype) than the control group (OR = 1.772, p = 0.007) (Table 3, Figure 3).
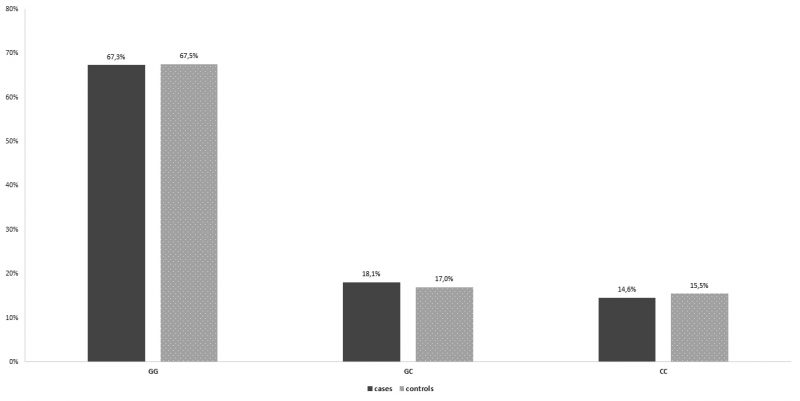
Figure 1. Genotype distribution for IL-6-634 per group.
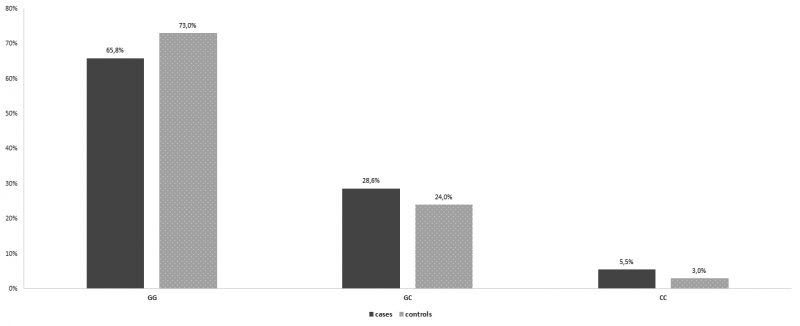
Figure 2. Genotype distribution for IL-6-174 per group.
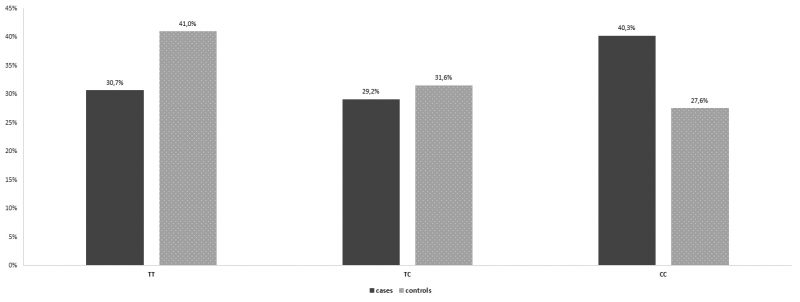
Figure 3. Genotype distribution for IL-1β-511 per group.
In the group of patients with recurrent spontaneous miscarriages, 102 women (51,3% ) were homozygous for the wild type allele, miR-149TT, 68 women (34,2%) were heterozygous, miR-149TC, while 29 women (14,6%) were revealed homozygous for the miR -149 T>C polymorphism, miR-499CC. The frequencies of miR-499CC (OR= 0,896, Pvalue= 0,692, 95% Confidence Interval [CI] = 0,519 – 1,546) genotype were not higher in patients with at least two consecutive spontaneous miscarriages than control group and the difference was not statistically significant (Table 4, Figure 4). On the other hand, in the group of patients with recurrent spontaneous miscarriages, 58 women (29,1%) were homozygous for the wild type allele, miR-27aAA, 90 women (45,2%) were heterozygous, miR-27aAG, while 51 women (25,6% were revealed homozygous for the miR-27aA>G polymorphism, miR-27a GG. The frequencies of miR-27a GG (OR= 3,283, Pvalue<0,001, 95% Confidence Interval [CI] = 1,857 – 5,804) genotype were higher in patients with at least two consecutive spontaneous miscarriages than control group and the difference was statistically significant. In specific, patients with recurrent abortions are 3.2 times statistically significantly more likely to have polymorphism (GG) compared to controls (OR = 3.283, p <0.001) (Table 5, Figure 5).
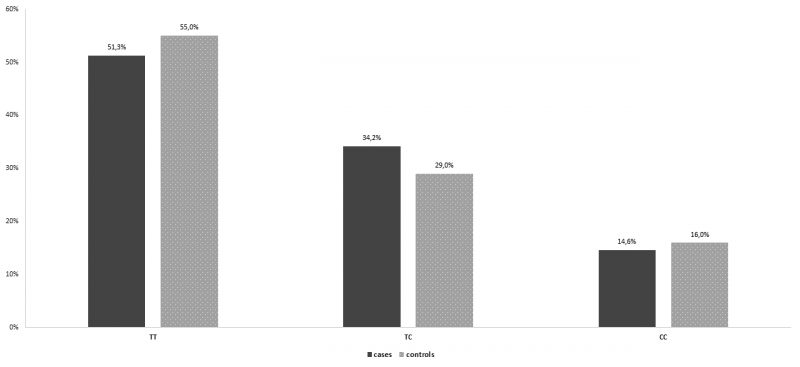
Figure 4. Genotype distribution for miR-149 per group.
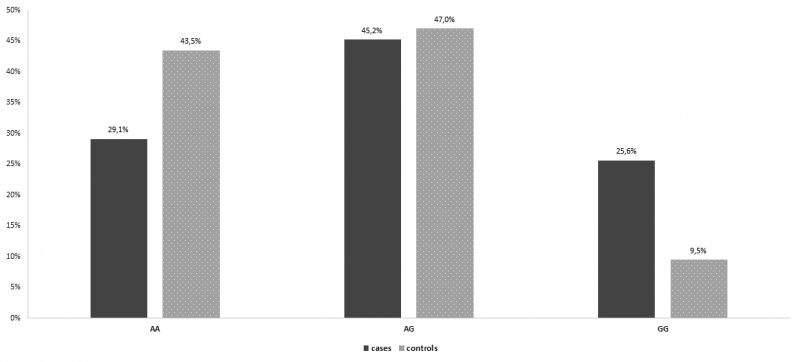
Figure 5. Genotype distribution for miR-27 per group.
Statistical Inference
Of the 5 genes tested, genotype and group correlation was found in IL-1b-511 and miR-27.
Specifically :
A) In the IL-1b-511 gene, cases have a 77.2% statistically significantly higher relative probability of having the polymorphism (CC genotype) than the control group (OR = 1.772, p = 0.007).
B) In the miR-27 gene, cases are 3.2 times statistically significantly more likely to have polymorphism (GG) compared to controls (OR = 3.283, p <0.001).
Discussion
Our results showed an association between IL1β – 511CC and recurrent abortions. Several studies have shown an increased risk of miscarriage. According to a study conducted by Wang and colleagues, IL1β – 511CC polymorphism has been associated with susceptibility to recurrent miscarriages (Th1 trophoblast immunity) in the Caucasian population26. Moreover, a meta-analysis by Zhang and colleagues in 2017 (21 scientific studies) showed that IL – 1β 511T / C polymorphism is consistently correlated with RPLs27. In addition, Ma and colleagues in 2017 also showed that IL1β-511CC polymorphism is associated with an increased risk of recurrent miscarriages (OR = 1,377) in the Chinese population23. Also, Alkhuriji and colleagues in 2020 showed a statistically significant difference in the frequency of TT genotype in women from Saudi Arabia with RPL without PCOS (p = 0, 047)28.
On the contrary, there are studies that have not associated this polymorphism with subsequent miscarriages. In specific, Linjawi and colleagues in 2005 showed that there is no correlation between that polymorphism and recurrent miscarriages in the Caucasian population29. In addition, a systematic Review by Bombell and McGuire in 2008 showed no statistically significant correlation of that polymorphism with RPLs30. Similarly, the same year, Choi and Kwak-Kim in a review concluded that it is quite unlikely that a single polymorphism is the main cause of RPL31. Ma et al., 2011 showed no statistically significant correlation of polymorphism with RPLs in Chinese population32. A study by Ali Rahmani in 2018 did not led to statistically significant differences between alleles and genotypic frequencies. IL1β-511T / C polymorphism may not be involved or play a functional role in recurrent abortions in the Iranian population33. However, in our study, we did not find a statistically significant association between IL6 -634 and IL-6 -174 genotypes and RPL risk. On the contrary, recent studies have shown that there is an association between the above genotypes and the risk of PRL. In specific, Zhang and colleagues in 2017 via a meta-analysis (3 articles, 303 RPL patients and 314 controls), showed that there is an increased risk of recurrent miscarriages in the group of IL – 6 634 G > C (rs1800796) (p <0.0001, OR = 2.91)27. Similarly, Salimi and colleagues in 2020 through a meta-analysis (7 studies, 1523 patients and 1554 controls) showed an association of IL – 6 634 G > C polymorphism with increased risk for RPL20.
Moreover, a statistically significant difference has unraveled regarding the AND miR-27aA>G gene polymorphism. The effects of miRNA polymorphisms on pregnancy loss have been reported in a limited number of studies. Functional analyses indicated that the variant genotypes of miR-27a, AG, and GG might be responsible for the elevated miR-27a levels34. Similarly, a recent case control study conducted by Rah and colleagues involving 225 controls and 387 women with at least two consecutively recurrent pregnancy losses between 1999 and 2012 showed associations between miRNA polymorphisms (miR-27a rs895819 and miR-449b rs10061133) and RPL development, and between the miRNA polymorphism (miR-27a rs895819) and plasma folate levels. However, in our study, we did not find a statistically significant association between miR-149 genotypes and RPL risk.
Conclusions
The above results are of major importance, as they propose a genetic panel related to recurrent abortions. Consequently gynecologists and researchers may apply it to establish a personalized treatment in women with RPL. The establishment of genetic panels related to infertility issues leads to more efficient solutions for the achievement of a pregnancy.
Supporting agency(ies): This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers – 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (IKY).
References
1. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108:393–406.
2. Bender Atik R, Bjarne Christiansen O, Elson J, Marie Kolte A, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N, Goddijn M. ESHRE guideline: recurrent pregnancy loss, 2018. Human Reprod Open. 2018;2:1–12.
3. Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579–587.
4. Kim J.O., Lee W.S., Lee B.E., Jeon Y.J., Kim Y.R., Jung S.H., Chang S.W., Kim N.K. Interleukin-1beta -511T>C genetic variant contributes to recurrent pregnancy loss risk and peripheral natural killer cell proportion. Fertil. Steril. 2014;102:206–212.
5. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Women’s Health. 2017;9:331–345.
6. Sugiura-Ogasawara M, Ozaki Y, Suzumori N. Management of recurrent miscarriage. J Obstet Gynaecol Res. 2014;40:1174–1179.
7. Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–317.
8. Page JM, Silver RM. Genetic causes of recurrent pregnancy loss. Clin Obstet Gynecol. 2016;59:498–508.
9. Kim J.H., Jeon Y.J., Lee B.E., Kang H., Shin J.E., Choi D.H., Lee W.S.L., Kim N.K. Association of methionine synthase and thymidylate synthase genetic polymorphisms with idiopathic recurrent pregnancy loss. Fertil. Steril. 2013;99:1674–1680.
10. Lee H.A., Ahn E.H., Kim J.H., Kim J.O., Ryu C.S., Lee J.Y., Cho S.H., Lee W.S., Kim N.K. Association study of frameshift and splice variant polymorphisms with risk of idiopathic recurrent pregnancy loss. Mol. Med. Rep. 2018;18:2417–2426.
11. Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009; 136:215–233.
12. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
13. Tüfekci KU, Meuwissen RL, Genç S. The role of microRNAs in biological processes. Methods Mol Biol. 2014;1107:15–31.
14. Tesfaye D, Salilew-Wondim D, Gebremedhn S, Sohel MM, Pandey HO, Hoelker M, Schellander K. Potential role of microRNAs in mammalian female fertility. Reprod Fertil Dev. 2016;29:8–23.
15. Liu W, Niu Z, Li Q, Pang RT, Chiu PC, Yeung WS. MicroRNA and embryo implantation. Am J Reprod Immunol. 2016;75:263–2671.
16. Liang J, Wang S, Wang Z. Role of microRNAs in embryo implantation. Reprod Biol Endocrinol. 2017;15:90
17. Alipour M, Abtin M, Hosseinzadeh A, Maleki M. Association between miR-146a C > G, miR-149 T > C, miR-196a2 T > C, and miR-499 A > G polymorphisms and susceptibility to idiopathic recurrent pregnancy loss. J Assist Reprod Genet. 2019 ;36:2237-2244.
18. Rah H, Chung KW, Ko KH, et al. miR-27a and miR-449b polymorphisms associated with a risk of idiopathic recurrent pregnancy loss. PLoS One. 2017;12:e0177160.
19. Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H. A survey on the role of interleukin-10 in breast cancer: a narrative. Rep Biochem Mol Biol. 2018;7:30–37.
20. Salimi E, Karimi-Zarchi M, Dastgheib SA, Abbasi H, Tabatabaiee RS, Hadadan A, Amjadi N, Akbarian-Bafghi MJ, Neamatzadeh H. Association of Promoter Region Polymorphisms of IL-6 and IL-18 Genes with Risk of Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis. Fetal Pediatr Pathol. 2020;39:346-359.
21. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095-2147.
22. Sobti RC, Kordi Tamandani DM, Shekari M, Kaur P, Malekzadeh K, Suri V. Interleukin 1 beta gene polymorphism and risk of cervical cancer. Int J Gynaecol Obstet. 2008;101:47-52.
23. Ma J, Zhang X, He G, Yang C. Association between TNF, IL1B, IL6, IL10 and IFNG polymorphisms and recurrent miscarriage: a case control study. Reprod Biol Endocrinol. 2017;15:83.
24. Wang T, Lu N, Cui Y, Tian L. Polymorphisms in interleukin genes and their association with the risk of recurrent pregnancy loss. Genes Genet Syst. 2019;94:109-116.
25. L Bohiltea C, E Radoi V. Interleukin-6 and interleukin-10 gene polymorphisms and recurrent pregnancy loss in Romanian population. Iran J Reprod Med. 2014;12:617-622.
26. Wang B, Feliciani C, Howell BG, et al. Contribution of Langerhans cell-derived IL-18 to contact hypersensitivity. J Immunol. 2002;168:3303-3308.
27. Zhang M, Xu J, Bao X, et al. Association between Genetic Polymorphisms in Interleukin Genes and Recurrent Pregnancy Loss – A Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0169891.
28. Alkhuriji AF, Al Omar SY, Babay ZA, et al. Association of IL-1β, IL-6, TNF-α, and TGFβ1 Gene Polymorphisms with Recurrent Spontaneous Abortion in Polycystic Ovary Syndrome. Dis Markers. 2020;2020:6076274.
29. Linjawi S, Li TC, Laird S, Blakemore A. Interleukin-1 receptor antagonist and interleukin-1 beta polymorphisms in women with recurrent miscarriage. Fertil Steril. 2005;83:1549-1552.
30. Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: meta-analysis. Aust N Z J Obstet Gynaecol. 2008;48:147-154.
31. Choi YK, Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol. 2008;60:91-110.
32. Ma X, Xu LJ, Wang J, Xian MM, Liu M. Association of IL-1β and IL-6 gene polymorphisms with recurrent spontaneous abortion in a Chinese Han population. Int J Immunogenet. 2012;39:15-19.
33. Ali Rahmani S, Paknejad Z, Mohammadkhanlou M, Daneshparvar M. Association of of IL-1 receptor antagonist (IL-1RN) and interleukin-1β genes (IL-1β) polymorphisms with recurrent pregnancy loss in Iranian Azeri women. Horm Mol Biol Clin Investig. 2017;33(3):/j/hmbci.2018.33.issue-3/hmbci-2017-0044/hmbci-2017-0044.xml.
34. Sun Q, Gu H, Zeng Y, Xia Y, Wang Y, Jing Y, et al. Hsa-mir-27a genetic variant contributes to gastric cancer susceptibility through affecting miR-27a and target gene expression. Cancer Sci. 2010;101:2241–7.